Structural Basis for Inhibition of Eg5 by Dihydropyrimidines: Stereoselectivity of Antimitotic Inhibitors Enastron, Dimethylenastron and Fluorastrol.
Kaan, H.Y.K., Ulaganathan, V., Rath, O., Prokopcova, H., Dallinger, D., Kappe, C.O., Kozielski, F.(2010) J Med Chem 53: 5676
- PubMed: 20597485
- DOI: https://doi.org/10.1021/jm100421n
- Primary Citation of Related Structures:
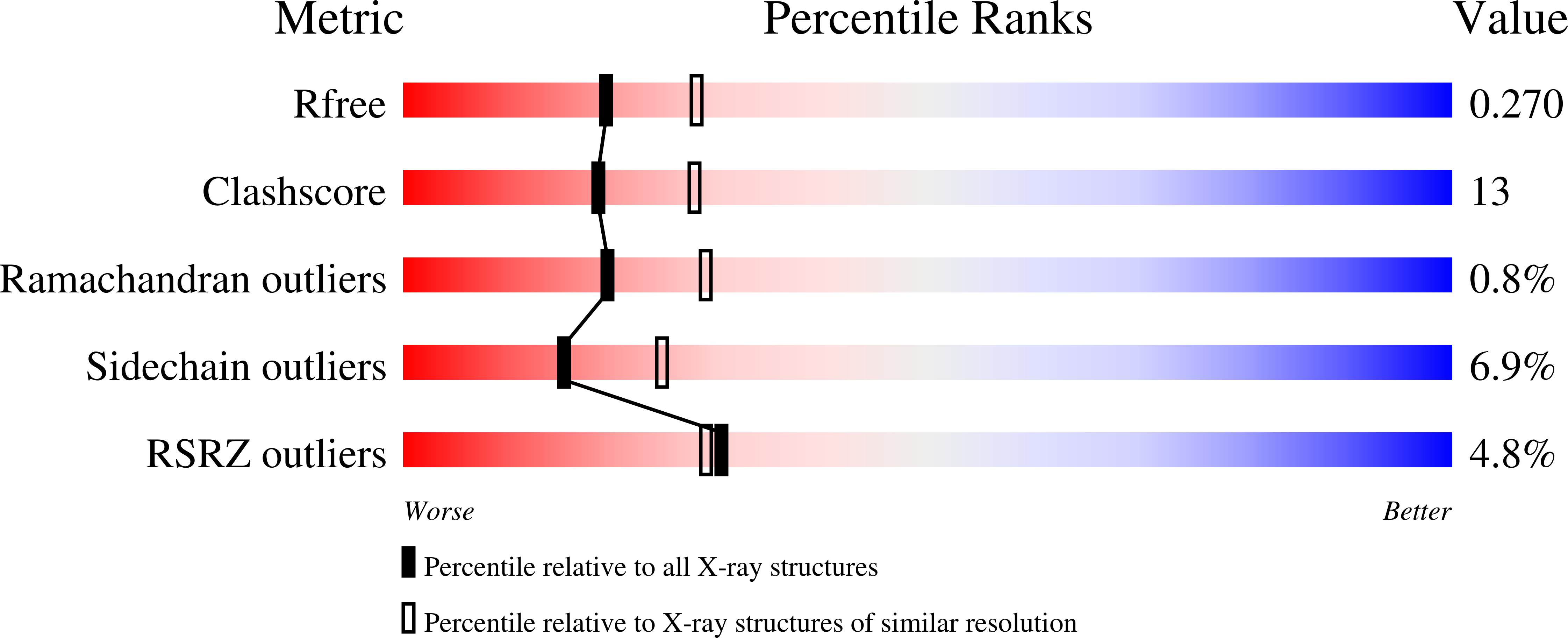
2X7C, 2X7D, 2X7E - PubMed Abstract:
Human kinesin Eg5, which plays an essential role in mitosis by establishing the bipolar spindle, has proven to be an interesting drug target for the development of cancer chemotherapeutics. Here, we report the crystal structures of the Eg5 motor domain complexed with enastron, dimethylenastron, and fluorastrol. By comparing these structures to that of monastrol and mon-97, we identified the main reasons for increased potency of these new inhibitors, namely the better fit of the ligand to the allosteric binding site and the addition of fluorine atoms. We also noticed preferential binding of the S-enantiomer of enastron and dimethylenastron to Eg5, while the R-enantiomer of fluorastrol binds preferentially to Eg5. In addition, we performed a multidrug resistance (MDR) study in cell lines overexpressing P-glycoprotein (Pgp). We showed that one of these inhibitors may have the potential to overcome susceptibility to this efflux pump and hence overcome common resistance associated with tubulin-targeting drugs.
Organizational Affiliation:
The Beatson Institute for Cancer Research, Garscube Estate, Bearsden, Glasgow, Scotland, UK.