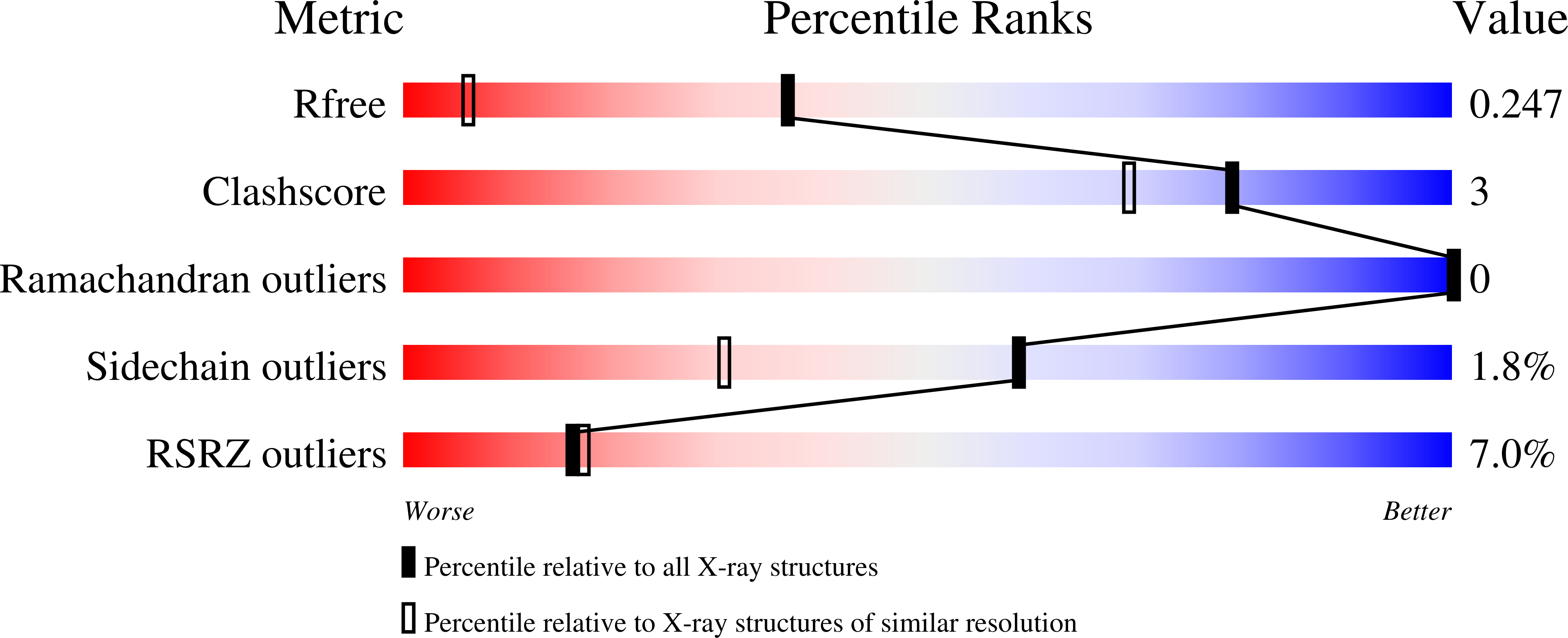
A Single Amino Acid Mutation in Zebrafish (Danio rerio) Liver Bile Acid-binding Protein Can Change the Stoichiometry of Ligand Binding.
Capaldi, S., Guariento, M., Saccomani, G., Fessas, D., Perduca, M., Monaco, H.L.(2007) J Biol Chem 282: 31008-31018
- PubMed: 17670743
- DOI: https://doi.org/10.1074/jbc.M705399200
- Primary Citation of Related Structures:
2QO4, 2QO5, 2QO6 - PubMed Abstract:
In all of the liver bile acid-binding proteins (L-BABPs) studied so far, it has been found that the stoichiometry of binding is of two cholate molecules per internal binding site. In this paper, we describe the expression, purification, crystallization, and three-dimensional structure determination of zebrafish (Danio rerio) L-BABP to 1.5A resolution, which is currently the highest available for a protein of this family. Since we have found that in zebrafish, the stoichiometry of binding in the protein cavity is of only one cholate molecule per wild type L-BABP, we examined the role of two crucial amino acids present in the binding site. Using site-directed mutagenesis, we have prepared, crystallized, and determined the three-dimensional structure of co-crystals of two mutants. The mutant G55R has the same stoichiometry of binding as the wild type protein, whereas the C91T mutant changes the stoichiometry of binding from one to two ligand molecules in the cavity and therefore appears to be more similar to the other members of the L-BABP family. Based on the presence or absence of a single disulfide bridge, it can be postulated that fish should bind a single cholate molecule, whereas amphibians and higher vertebrates should bind two. Isothermal titration calorimetry has also revealed the presence in the wild type protein and the G55R mutant of an additional binding site, different from the first and probably located on the surface of the molecule.
Organizational Affiliation:
Biocrystallography Laboratory, Department of Science and Technology, University of Verona, Ca Vignal 1, Strada Le Grazie 15, 37134 Verona, Italy.