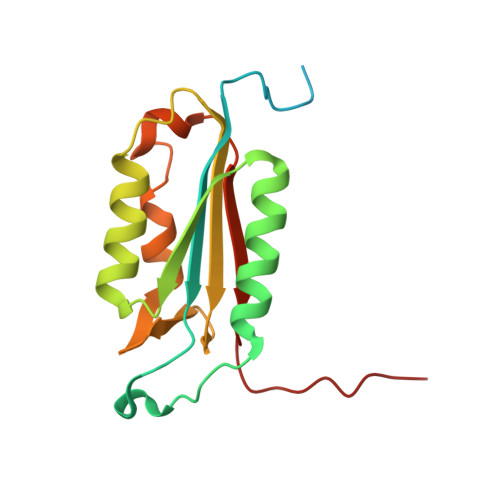
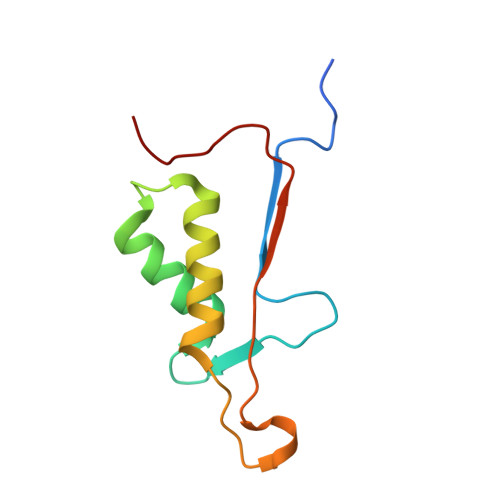
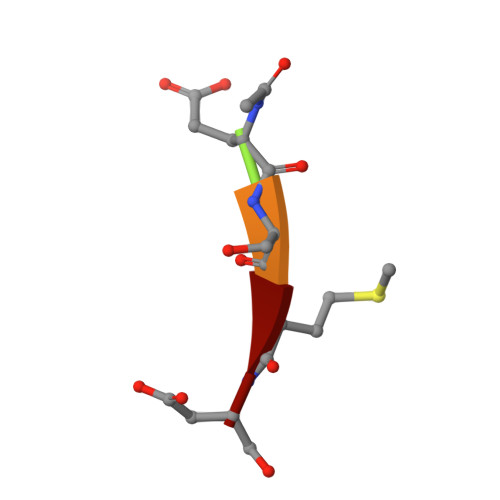
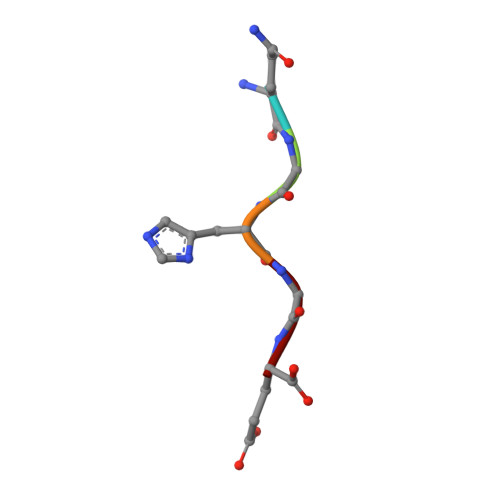
Plasticity of S2-S4 specificity pockets of executioner caspase-7 revealed by structural and kinetic analysis.
Agniswamy, J., Fang, B., Weber, I.T.(2007) FEBS J 274: 4752-4765
- PubMed: 17697120
- DOI: https://doi.org/10.1111/j.1742-4658.2007.05994.x
- Primary Citation of Related Structures:
2QL5, 2QL7, 2QL9, 2QLB, 2QLF, 2QLJ - PubMed Abstract:
Many protein substrates of caspases are cleaved at noncanonical sites in comparison to the recognition motifs reported for the three caspase subgroups. To provide insight into the specificity and aid in the design of drugs to control cell death, crystal structures of caspase-7 were determined in complexes with six peptide analogs (Ac-DMQD-Cho, Ac-DQMD-Cho, Ac-DNLD-Cho, Ac-IEPD-Cho, Ac-ESMD-Cho, Ac-WEHD-Cho) that span the major recognition motifs of the three subgroups. The crystal structures show that the S2 pocket of caspase-7 can accommodate diverse residues. Glu is not required at the P3 position because Ac-DMQD-Cho, Ac-DQMD-Cho and Ac-DNLD-Cho with varied P3 residues are almost as potent as the canonical Ac-DEVD-Cho. P4 Asp was present in the better inhibitors of caspase-7. However, the S4 pocket of executioner caspase-7 has alternate regions for binding of small branched aliphatic or polar residues similar to those of initiator caspase-8. The observed plasticity of the caspase subsites agrees very well with the reported cleavage of many proteins at noncanonical sites. The results imply that factors other than the P4-P1 sequence, such as exosites, contribute to the in vivo substrate specificity of caspases. The novel peptide binding site identified on the molecular surface of the current structures is suggested to be an exosite of caspase-7. These results should be considered in the design of selective small molecule inhibitors of this pharmacologically important protease.
Organizational Affiliation:
Department of Biology, Molecular Basis of Disease, Georgia State University, Atlanta, GA 30302, USA.