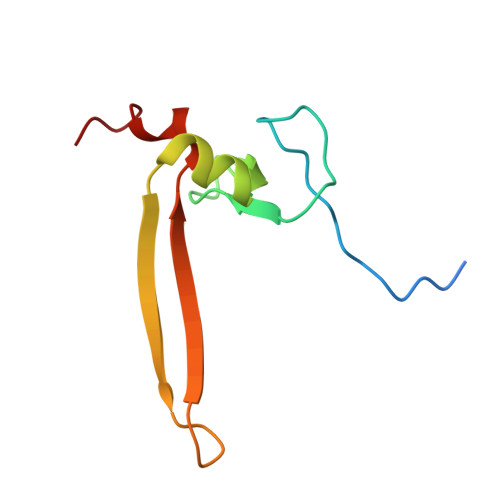
Solution structure of an atypical PHD finger in BRPF2 and its interaction with DNA
Liu, L., Qin, S., Zhang, J., Ji, P., Shi, Y., Wu, J.(2012) J Struct Biol 180: 165-173
- PubMed: 22820306
- DOI: https://doi.org/10.1016/j.jsb.2012.06.014
- Primary Citation of Related Structures:
2LQ6 - PubMed Abstract:
Plant homeodomain (PHD) finger is found to be a versatile reader that functions in recruiting transcription factors and chromatin modification complexes. Bromodomain- and PHD finger-containing (BRPF) proteins are identified as scaffold component in a couple of histone acetyltransferase (HATs) complexes but the biological function of PHD fingers, composing the motif called PZPM (PHD/Zn-knuckle/PHD Motif), in BRPF proteins is far from being well understood. Here we report the three-dimensional solution structure of the second PHD finger of PZPM in human BRPF2. According to the structure, BRPF2 PHD2 possesses a two-strand β sheet which is different from any other PHD fingers. Functionally, this PHD finger can potentially bind DNA non-specifically with an evolutionarily conserved and positively charged surface. We provide the structural and interaction information of this atypical PHD finger and categorize this BRPF2 PHD2 into a new subset of PHD finger. Moreover our work also shed light on the functional aspect of the PZPM.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230026, PR China.