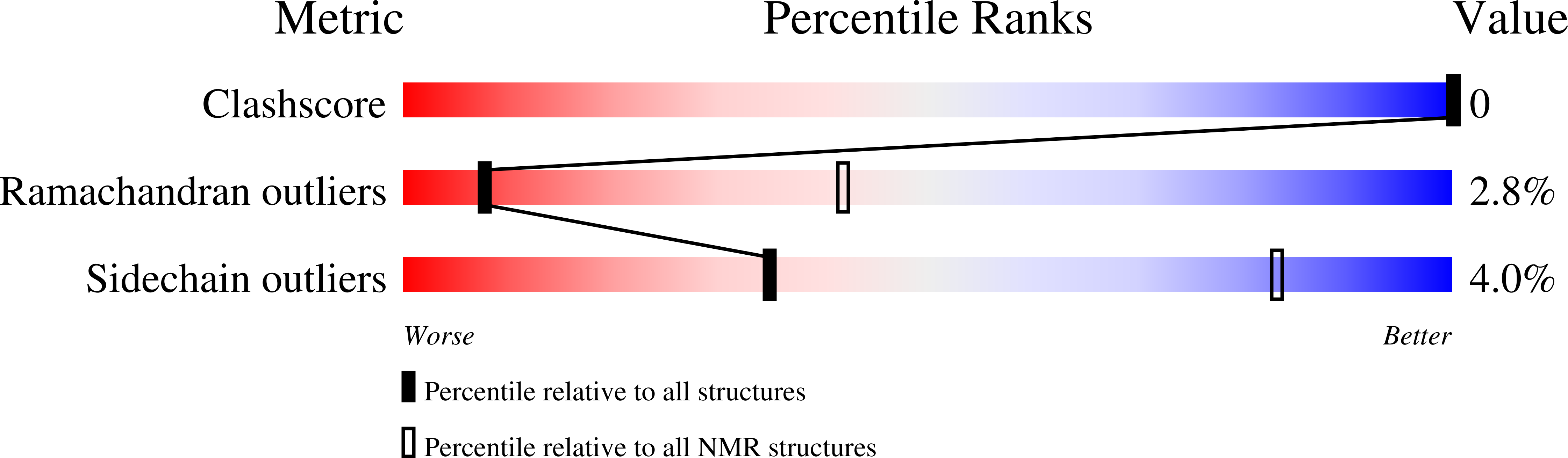Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide.
Forssmann, W.G., The, Y.H., Stoll, M., Adermann, K., Albrecht, U., Barlos, K., Busmann, A., Canales-Mayordomo, A., Gimenez-Gallego, G., Hirsch, J., Jimenez-Barbero, J., Meyer-Olson, D., Munch, J., Perez-Castells, J., Standker, L., Kirchhoff, F., Schmidt, R.E.(2010) Sci Transl Med 2: 63re3-63re3
- PubMed: 21178138
- DOI: https://doi.org/10.1126/scitranslmed.3001697
- Primary Citation of Related Structures:
2L6S, 2L6T - PubMed Abstract:
To infect host cells, most enveloped viruses must insert a hydrophobic fusion peptide into the host cell membrane. Thus, fusion peptides may be valuable targets for developing drugs that block virus entry. We have shown previously that a natural 20-residue fragment of α(1)-antitrypsin, designated VIRus-Inhibitory Peptide (VIRIP), that binds to the gp41 fusion peptide of HIV-1 prevents the virus from entering target cells in vitro. Here, we examine the efficacy of 10-day monotherapy with the optimized VIR-576 derivative of VIRIP in treatment-naïve, HIV-1-infected individuals with viral RNA loads of ≥10,000 copies per ml. We report that at the highest dose (5.0 grams per day), intravenous infusion of VIR-576 reduced the mean plasma viral load by 1.23 log(10) copies per ml without causing severe adverse effects. Our results are proof of concept that fusion peptide inhibitors suppress viral replication in human patients, and offer prospects for the development of a new class of drugs that prevent virus particles from anchoring to and infecting host cells.
Organizational Affiliation:
Department of Immunology and Rheumatology, Hannover Medical University, Carl-Neuberg-Strasse 1, D-30625 Hannover, Germany.