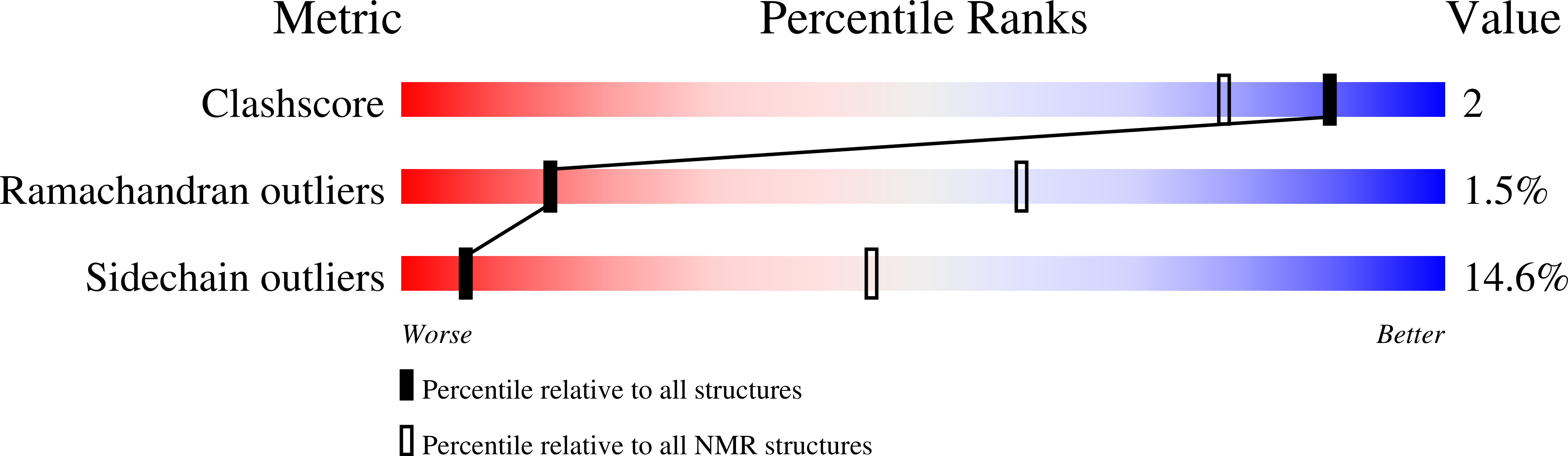Comparison of NMR and crystal structures highlights conformational isomerism in protein active sites.
Serrano, P., Pedrini, B., Geralt, M., Jaudzems, K., Mohanty, B., Horst, R., Herrmann, T., Elsliger, M.A., Wilson, I.A., Wuthrich, K.(2010) Acta Crystallogr Sect F Struct Biol Cryst Commun 66: 1393-1405
- PubMed: 20944236
- DOI: https://doi.org/10.1107/S1744309110033658
- Primary Citation of Related Structures:
2KA5, 2KL2 - PubMed Abstract:
The JCSG has recently developed a protocol for systematic comparisons of high-quality crystal and NMR structures of proteins. In this paper, the extent to which this approach can provide function-related information on the two functionally annotated proteins TM1081, a Thermotoga maritima anti-σ factor antagonist, and A2LD1 (gi:13879369), a mouse γ-glutamylamine cyclotransferase, is explored. The NMR structures of the two proteins have been determined in solution at 313 and 298 K, respectively, using the current JCSG protocol based on the software package UNIO for extensive automation. The corresponding crystal structures were solved by the JCSG at 100 K and 1.6 Å resolution and at 100 K and 1.9 Å resolution, respectively. The NMR and crystal structures of the two proteins share the same overall molecular architectures. However, the precision of the structure determination along the amino-acid sequence varies over a significantly wider range in the NMR structures than in the crystal structures. Thereby, in each of the two NMR structures about 65% of the residues have displacements below the average and in both proteins the less well ordered residues include large parts of the active sites, in addition to some highly solvent-exposed surface areas. Whereas the latter show increased disorder in the crystal and in solution, the active-site regions display increased displacements only in the NMR structures, where they undergo local conformational exchange on the millisecond time scale that appears to be frozen in the crystals. These observations suggest that a search for molecular regions showing increased structural disorder and slow dynamic processes in solution while being well ordered in the corresponding crystal structure might be a valid initial step in the challenge of identifying putative active sites in functionally unannotated proteins with known three-dimensional structure.
Organizational Affiliation:
Department of Molecular Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.