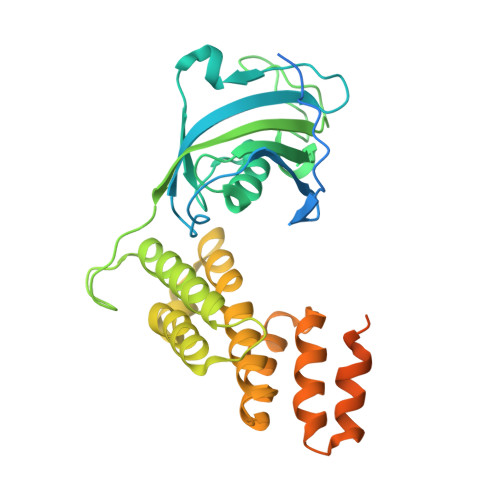
Crystal Structure of a Multi-domain Immunophilin from Arabidopsis thaliana: A Paradigm for Regulation of Plant ABC Transporters.
Granzin, J., Eckhoff, A., Weiergraber, O.H.(2006) J Mol Biol 364: 799-809
- PubMed: 17045295
- DOI: https://doi.org/10.1016/j.jmb.2006.09.052
- Primary Citation of Related Structures:
2IF4 - PubMed Abstract:
FKBP42 is a membrane-anchored immunophilin playing a critical role in morphogenesis and development of higher plants. We present the X-ray structure of the cytoplasmic portion of FKBP42 comprising both the FKBP-like domain and the TPR domain at 2.85 A resolution. The data shed light on the probable binding modes of key interaction partners, including HSP90 and two classes of ABC transporters. The resulting models provide a structural background for further investigation of the unique biological properties of this protein.
Organizational Affiliation:
Institute of Neurosciences and Biophysics, Molecular Biophysics, Research Centre Jülich, D-52425 Jülich, Germany.