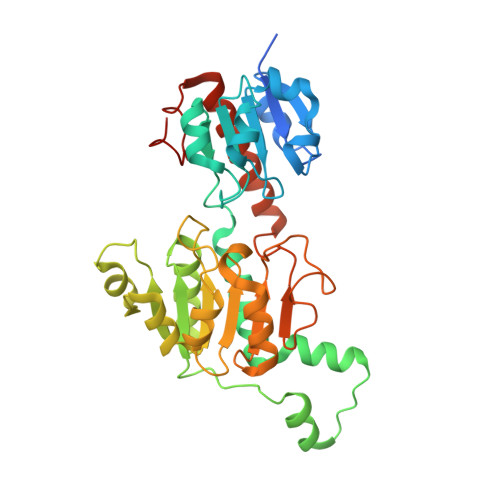
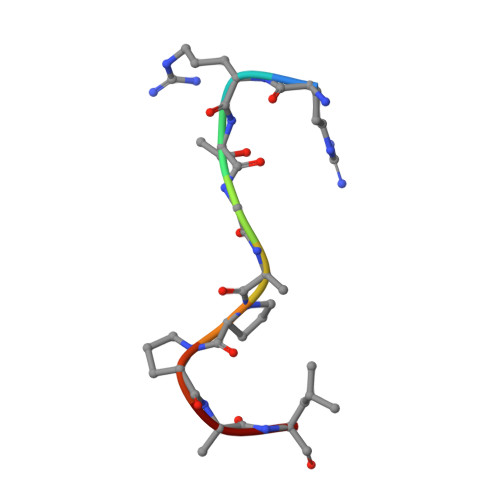
Specific Recognition of ZNF217 and Other Zinc Finger Proteins at a Surface Groove of C-Terminal Binding Proteins
Quinlan, K.G.R., Nardini, M., Verger, A., Francescato, P., Yaswen, P., Corda, D., Bolognesi, M., Crossley, M.(2006) Mol Cell Biol 26: 8159-8172
- PubMed: 16940172
- DOI: https://doi.org/10.1128/MCB.00680-06
- Primary Citation of Related Structures:
2HU2 - PubMed Abstract:
Numerous transcription factors recruit C-terminal binding protein (CtBP) corepressors. We show that the large zinc finger protein ZNF217 contacts CtBP. ZNF217 is encoded by an oncogene frequently amplified in tumors. ZNF217 contains a typical Pro-X-Asp-Leu-Ser (PXDLS) motif that binds in CtBP's PXDLS-binding cleft. However, ZNF217 also contains a second motif, Arg-Arg-Thr (RRT), that binds a separate surface on CtBP. The crystal structure of CtBP bound to an RRTGAPPAL peptide shows that it contacts a surface crevice distinct from the PXDLS binding cleft. Interestingly, both PXDLS and RRT motifs are also found in other zinc finger proteins, such as RIZ. Finally, we show that ZNF217 represses several promoters, including one from a known CtBP target gene, and mutations preventing ZNF217's contact with CtBP reduce repression. These results identify a new CtBP interaction motif and establish ZNF217 as a transcriptional repressor protein that functions, at least in part, by associating with CtBP.
Organizational Affiliation:
School of Molecular and Microbial Biosciences, University of Sydney, Sydney, NSW 2006, Australia.