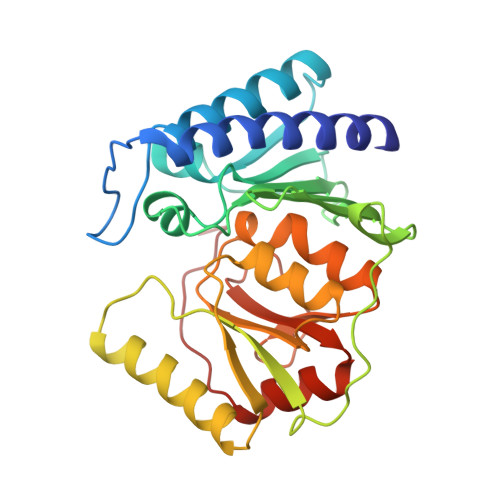
Structure of inositol monophosphatase, the putative target of lithium therapy.
Bone, R., Springer, J.P., Atack, J.R.(1992) Proc Natl Acad Sci U S A 89: 10031-10035
- PubMed: 1332026
- DOI: https://doi.org/10.1073/pnas.89.21.10031
- Primary Citation of Related Structures:
2HHM - PubMed Abstract:
Inositol monophosphatase (EC 3.1.3.25), the putative molecular site of action of lithium therapy for manic-depressive illness, plays a key role in the phosphatidylinositol signaling pathway by catalyzing the hydrolysis of inositol monophosphates. To provide a structural basis from which to design better therapeutic agents for manic-depressive illness, the structure of human inositol monophosphatase has been determined to 2.1-A resolution by using x-ray crystallography. The enzyme exists as a dimer of identical subunits, each folded into a five-layered sandwich of three pairs of alpha-helices and two beta-sheets. Sulfate and an inhibitory lanthanide cation (Gd3+) are bound at identical sites on each subunit and establish the positions of the active sites. Each site is located in a large hydrophilic cavern that is at the base of the two central helices where several segments of secondary structure intersect. Comparison of the phosphatase aligned sequences of several diverse genes with the phosphatase structure suggests that the products of these genes and the phosphatase form a structural family with a conserved metal binding site.
Organizational Affiliation:
Department of Biophysical Chemistry, Merck Research Laboratories, Rahway, NJ 07065.