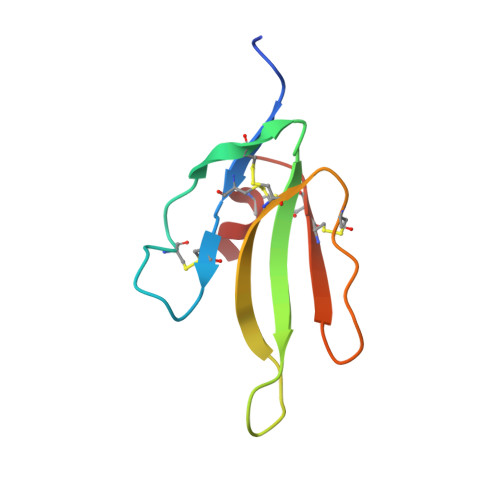
Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity.
Pawlak, J., Mackessy, S.P., Fry, B.G., Bhatia, M., Mourier, G., Fruchart-Gaillard, C., Servent, D., Menez, R., Stura, E., Menez, A., Kini, R.M.(2006) J Biol Chem 281: 29030-29041
- PubMed: 16864572
- DOI: https://doi.org/10.1074/jbc.M605850200
- Primary Citation of Related Structures:
2H5F - PubMed Abstract:
Boiga dendrophila (mangrove catsnake) is a colubrid snake that lives in Southeast Asian lowland rainforests and mangrove swamps and that preys primarily on birds. We have isolated, purified, and sequenced a novel toxin from its venom, which we named denmotoxin. It is a monomeric polypeptide of 77 amino acid residues with five disulfide bridges. In organ bath experiments, it displayed potent postsynaptic neuromuscular activity and irreversibly inhibited indirectly stimulated twitches in chick biventer cervicis nerve-muscle preparations. In contrast, it induced much smaller and readily reversible inhibition of electrically induced twitches in mouse hemidiaphragm nerve-muscle preparations. More precisely, the chick muscle alpha(1)betagammadelta-nicotinic acetylcholine receptor was 100-fold more susceptible compared with the mouse receptor. These data indicate that denmotoxin has a bird-specific postsynaptic activity. We chemically synthesized denmotoxin, crystallized it, and solved its crystal structure at 1.9 A by the molecular replacement method. The toxin structure adopts a non-conventional three-finger fold with an additional (fifth) disulfide bond in the first loop and seven additional residues at its N terminus, which is blocked by a pyroglutamic acid residue. This is the first crystal structure of a three-finger toxin from colubrid snake venom and the first fully characterized bird-specific toxin. Denmotoxin illustrates the relationship between toxin specificity and the primary prey type that constitutes the snake's diet.
Organizational Affiliation:
Department of Biological Sciences, Faculty of Science, National University of Singapore.