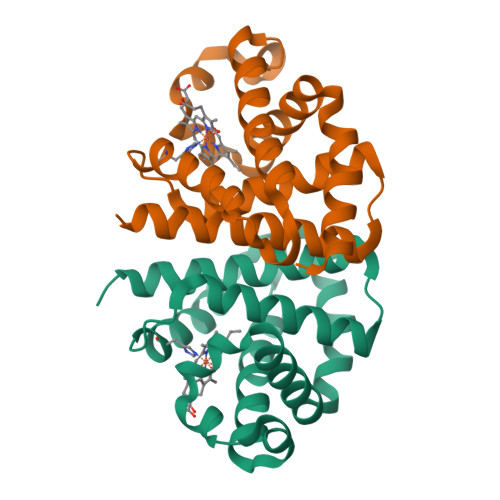
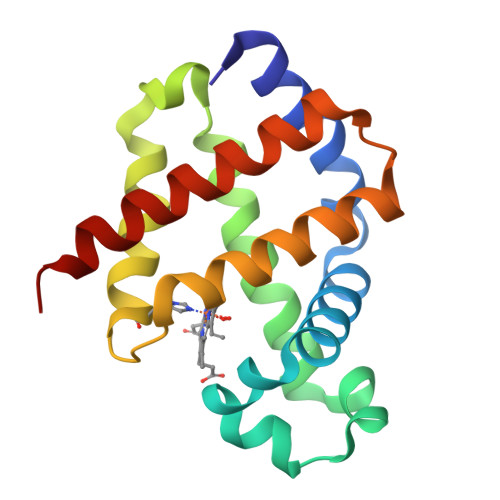
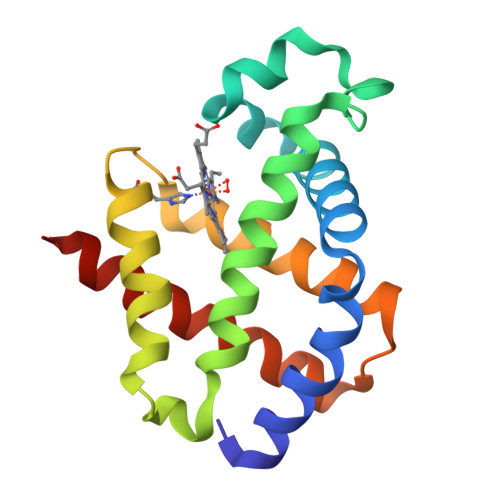
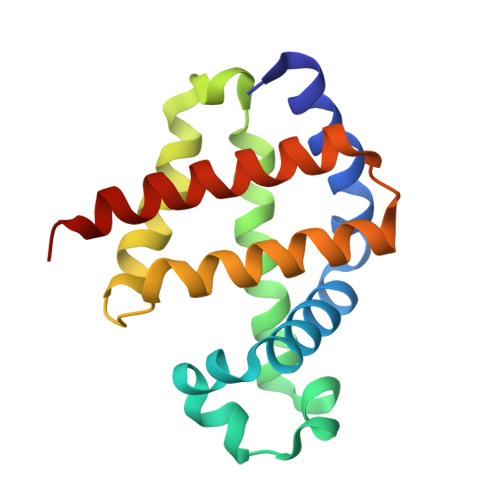
Modulation of Oxygen Binding to Insect Hemoglobins: The Structure of Hemoglobin from the Botfly Gasterophilus Intestinalis.
Pesce, A., Nardini, M., Dewilde, S., Hoogewijs, D., Ascenzi, P., Moens, L., Bolognesi, M.(2005) Protein Sci 14: 3057
- PubMed: 16260762
- DOI: https://doi.org/10.1110/ps.051742605
- Primary Citation of Related Structures:
2C0K - PubMed Abstract:
Hemoglobins (Hbs) reversibly bind gaseous diatomic ligands (e.g., O2) as the sixth heme axial ligand of the penta-coordinate deoxygenated form. Selected members of the Hb superfamily, however, display a functionally relevant hexa-coordinate heme Fe atom in their deoxygenated state. Endogenous heme hexa-coordination is generally provided in these Hbs by the E7 residue (often His), which thus modulates accessibility to the heme distal pocket and reactivity of the heme toward exogenous ligands. Such a pivotal role of the E7 residue is prominently shown by analysis of the functional and structural properties of insect Hbs. Here, we report the 2.6 A crystal structure of oxygenated Gasterophilus intestinalis Hb1, a Hb known to display a penta-coordinate heme in the deoxygenated form. The structure is analyzed in comparison with those of Drosophila melanogaster Hb, exhibiting a hexa-coordinate heme in its deoxygenated derivative, and of Chironomus thummi thummi HbIII, which displays a penta-coordinate heme in the deoxygenated form. Despite evident structural differences in the heme distal pockets, the distinct molecular mechanisms regulating O2 binding to the three insect Hbs result in similar O(2 affinities (P50 values ranging between 0.12 torr and 0.46 torr).
Organizational Affiliation:
Department of Biomolecular Sciences and Biotechnology, University of Milano, Via Celoria 26, I-20131 Milano, Italy.