The Effect of Replacing the Axial Methionine Ligand with a Lysine Residue in Cytochrome C-550 from Paracoccus Versutus Assessed by X-Ray Crystallography and Unfolding.
Worrall, J.A.R., Van Roon, A.-M.M., Ubbink, M., Canters, G.W.(2005) FEBS J 272: 2441
- PubMed: 15885094
- DOI: https://doi.org/10.1111/j.1742-4658.2005.04664.x
- Primary Citation of Related Structures:
2BGV, 2BH4, 2BH5 - PubMed Abstract:
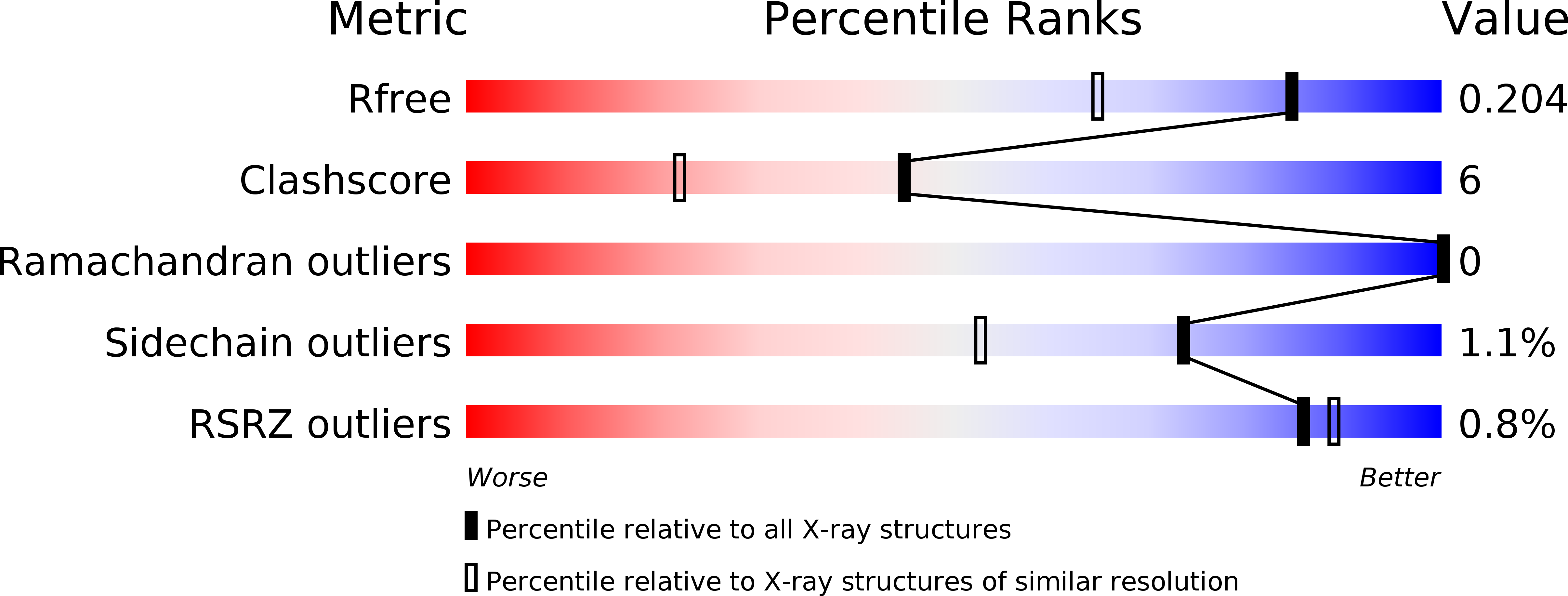
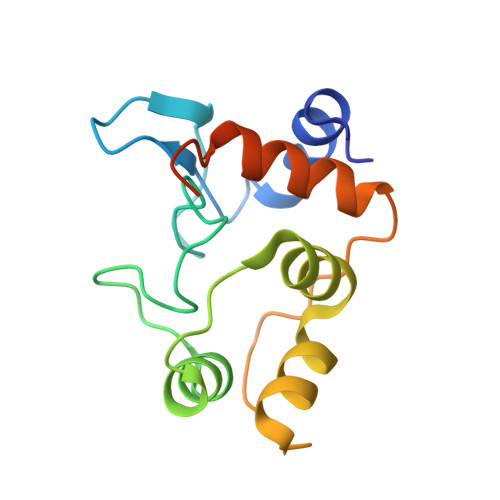
The structure of cytochrome c-550 from the nonphotosynthetic bacteria Paraccocus versutus has been solved by X-ray crystallography to 1.90 A resolution, and reveals a high structural homology to other bacterial cytochromes c(2). The effect of replacing the axial heme-iron methionine ligand with a lysine residue on protein structure and unfolding has been assessed using the M100K variant. From X-ray structures at 1.95 and 1.55 A resolution it became clear that the amino group of the lysine side chain coordinates to the heme-iron. Structural differences compared to the wild-type protein are confined to the lysine ligand loop connecting helices four and five. In the heme cavity an additional water molecule is found which participates in an H-bonding interaction with the lysine ligand. Under cryo-conditions extra electron density in the lysine ligand loop is revealed, leading to residues K97 to T101 being modeled with a double main-chain conformation. Upon unfolding, dissociation of the lysine ligand from the heme-iron is shown to be pH dependent, with NMR data consistent with the occurrence of a ligand exchange mechanism similar to that seen for the wild-type protein.
Organizational Affiliation:
Leiden Institute of Chemistry, Leiden University, Gorlaeus Laboratories, Leiden, the Netherlands.