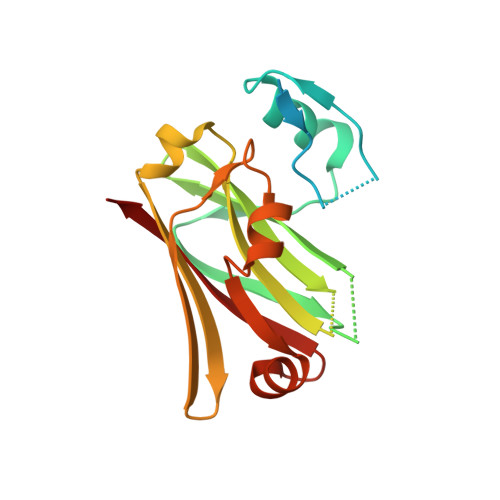
Structural Analysis of Siah1-Siah-interacting Protein Interactions and Insights into the Assembly of an E3 Ligase Multiprotein Complex
Santelli, E., Leone, M., Li, C., Fukushima, T., Preece, N.E., Olson, A.J., Ely, K.R., Reed, J.C., Pellecchia, M., Liddington, R.C., Matsuzawa, S.(2005) J Biol Chem 280: 34278-34287
- PubMed: 16085652
- DOI: https://doi.org/10.1074/jbc.M506707200
- Primary Citation of Related Structures:
2A25, 2A26 - PubMed Abstract:
Siah1 is the central component of a multiprotein E3 ubiquitin ligase complex that targets beta-catenin for destruction in response to p53 activation. The E3 complex comprises, in addition to Siah1, Siah-interacting protein (SIP), the adaptor protein Skp1, and the F-box protein Ebi. Here we show that SIP engages Siah1 by means of two elements, both of which are required for mediating beta-catenin destruction in cells. An N-terminal dimerization domain of SIP sits across the saddle-shaped upper surface of Siah1, with two extended legs packing against the sides of Siah1 by means of a consensus PXAXVXP motif that is common to a family of Siah-binding proteins. The C-terminal domain of SIP, which binds to Skp1, protrudes from the lower surface of Siah1, and we propose that this surface provides the scaffold for bringing substrate and the E2 enzyme into apposition in the functional complex.
Organizational Affiliation:
The Burnham Institute, La Jolla, California 92037, USA.