Mutational Study of Pseudopeptide Inhibitor Binding to HIV-1 Protease; Analysis of Four X-ray Structures
Duskova, J., Skalova, T., Dohnalek, J., Petrokova, H., Hasek, J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PROTEASE RETROPEPSIN | 99 | Human immunodeficiency virus 1 | Mutation(s): 3 EC: 3.4.23.16 | 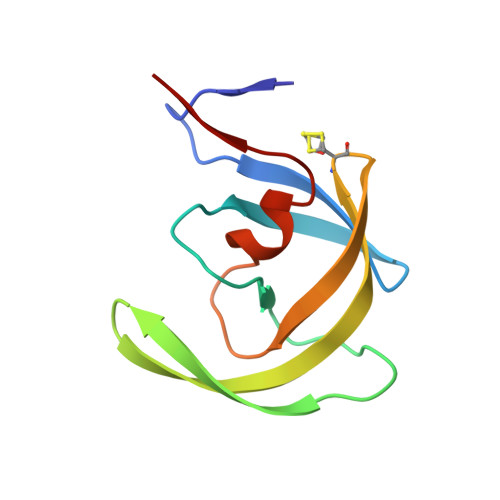 | |
UniProt | |||||
Find proteins for Q7ZCL6 (Human immunodeficiency virus 1) Explore Q7ZCL6 Go to UniProtKB: Q7ZCL6 | |||||
Find proteins for P03367 (Human immunodeficiency virus type 1 group M subtype B (isolate BRU/LAI)) Go to UniProtKB: P03367 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7ZCL6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 0ZS Query on 0ZS | C [auth A] | N-{(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-4-phenylbutyl}-L-phenylalanyl-L-alpha-glutamyl-L-phenylalaninamide C38 H49 N5 O8 MPMUDVMRFYJRLP-QEUNAIBPSA-N |  | ||
| CL Query on CL | D [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| NA Query on NA | E [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CME Query on CME | A, B | L-PEPTIDE LINKING | C5 H11 N O3 S2 |  | CYS |
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_000385 (0ZS) Query on PRD_000385 | C [auth A] | BOC-PHE-PSI[R-CH(OH)CH2NH]-PHE-GLU-PHE-NH2 | Peptide-like / Inhibitor |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 62.483 | α = 90 |
| b = 62.483 | β = 90 |
| c = 82.412 | γ = 120 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |
| SHELXL-97 | refinement |