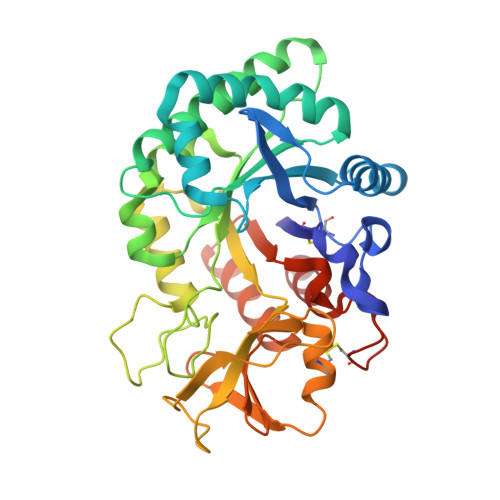
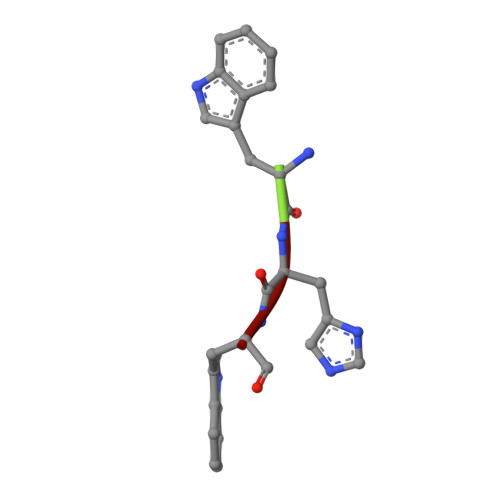
Crystal structure of the complex of signalling protein from sheep (SPS-40) with a designed peptide Trp-His-Trp reveals significance of Asn79 and Trp191 in the complex formation
Ethayathulla, A.S., Srivastava, D.B., Singh, N., Kumar, J., Somvanshi, R.K., Sharma, S., Dey, S., Singh, T.P.To be published.