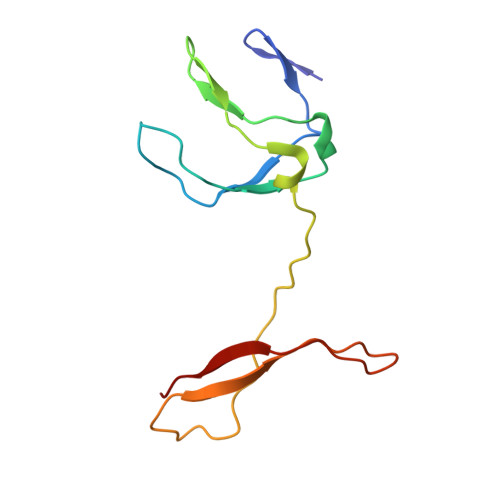
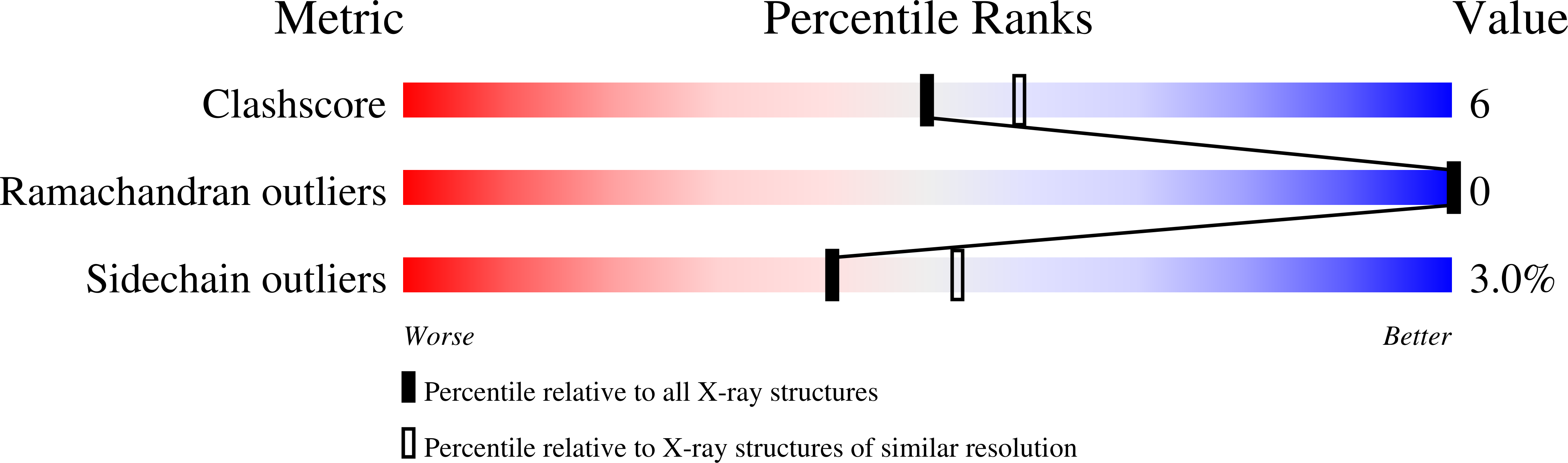NF-kappaB RelB forms an intertwined homodimer.
Huang, D.B., Vu, D., Ghosh, G.(2005) Structure 13: 1365-1373
- PubMed: 16154093
- DOI: https://doi.org/10.1016/j.str.2005.06.018
- Primary Citation of Related Structures:
1ZK9, 1ZKA - PubMed Abstract:
The X-ray structure of the RelB dimerization domain (DD) reveals that the RelBDD assumes an unexpected intertwined fold topology atypical of other NF-kappaB dimers. All typical NF-kappaB dimers are formed by the association of two independently folded immunoglobulin (Ig) domains. In RelBDD, two polypeptides reconstruct both Ig domains in the dimer with an extra beta sheet connecting the two domains. Residues most critical to NF-kappaB dimer formation are invariant in RelB, and Y300 plays a positive role in RelBDD dimer formation. The presence of RelB-specific nonpolar residues at the surface removes several intradomain surface hydrogen bonds that may render the domain fold unstable. Intertwining may stabilize the RelBDD homodimer by forming the extra beta sheet. We show that, as in the crystal, RelB forms an intertwined homodimer in solution. We suggest that the transiently stable RelB homodimer might prevent its rapid degradation, allowing for heterodimer formation with p50 and p52.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA.