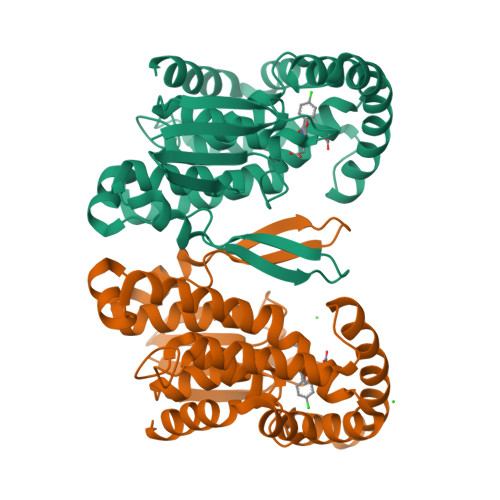
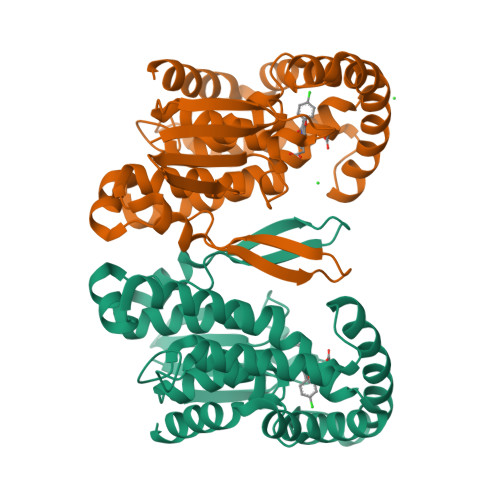
Crystal Structure and Possible Catalytic Mechanism of Microsomal Prostaglandin E Synthase Type 2 (mPGES-2).
Yamada, T., Komoto, J., Watanabe, K., Ohmiya, Y., Takusagawa, F.(2005) J Mol Biology 348: 1163-1176
- PubMed: 15854652
- DOI: https://doi.org/10.1016/j.jmb.2005.03.035
- Primary Citation of Related Structures:
1Z9H - PubMed Abstract:
Prostaglandin (PG) H(2) (PGH(2)), formed from arachidonic acid, is an unstable intermediate and is converted efficiently into more stable arachidonate metabolites (PGD(2), PGE(2), and PGF(2)) by the action of three groups of enzymes. Prostaglandin E synthase catalyzes an isomerization reaction, PGH(2) to PGE(2). Microsomal prostaglandin E synthase type-2 (mPGES-2) has been crystallized with an anti-inflammatory drug indomethacin (IMN), and the complex structure has been determined at 2.6A resolution. mPGES-2 forms a dimer and is attached to lipid membrane by anchoring the N-terminal section. Two hydrophobic pockets connected to form a V shape are located in the bottom of a large cavity. IMN binds deeply in the cavity by placing the OMe-indole and chlorophenyl moieties into the V-shaped pockets, respectively, and the carboxyl group interacts with S(gamma) of C110 by forming a H-bond. A characteristic H-bond chain formation (N-H...S(gamma)-H...S(gamma)...H-N) is seen through Y107-C113-C110-F112, which apparently decreases the pK(a) of S(gamma) of C110. The geometry suggests that the S(gamma) of C110 is most likely the catalytic site of mPGES-2. A search of the RCSB Protein Data Bank suggests that IMN can fit into the PGH(2) binding site in various proteins. On the basis of the crystal structure and mutation data, a PGH(2)-bound model structure was built. PGH(2) fits well into the IMN binding site by placing the alpha and omega-chains in the V-shaped pockets, and the endoperoxide moiety interacts with S(gamma) of C110. A possible catalytic mechanism is proposed on the basis of the crystal and model structures, and an alternative catalytic mechanism is described. The fold of mPGES-2 is quite similar to those of GSH-dependent hematopoietic prostaglandin D synthase, except for the two large loop sections.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, 1200 Sunnyside Ave, Lawrence, KS 66045-7534, USA.