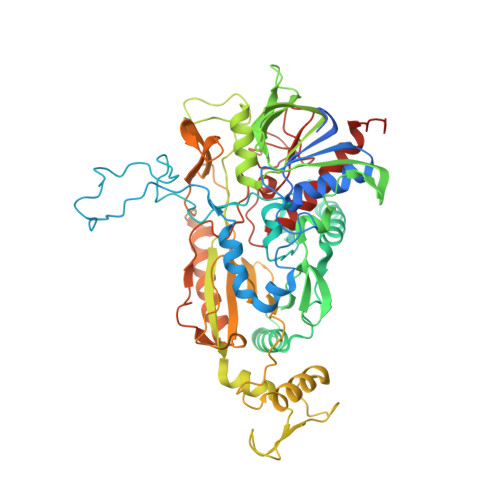
Crystal structure of the 270 kDa homotetrameric lignin-degrading enzyme pyranose 2-oxidase
Hallberg, B.M., Leitner, C., Haltrich, D., Divne, C.(2004) J Mol Biol 341: 781-796
- PubMed: 15288786
- DOI: https://doi.org/10.1016/j.jmb.2004.06.033
- Primary Citation of Related Structures:
1TT0 - PubMed Abstract:
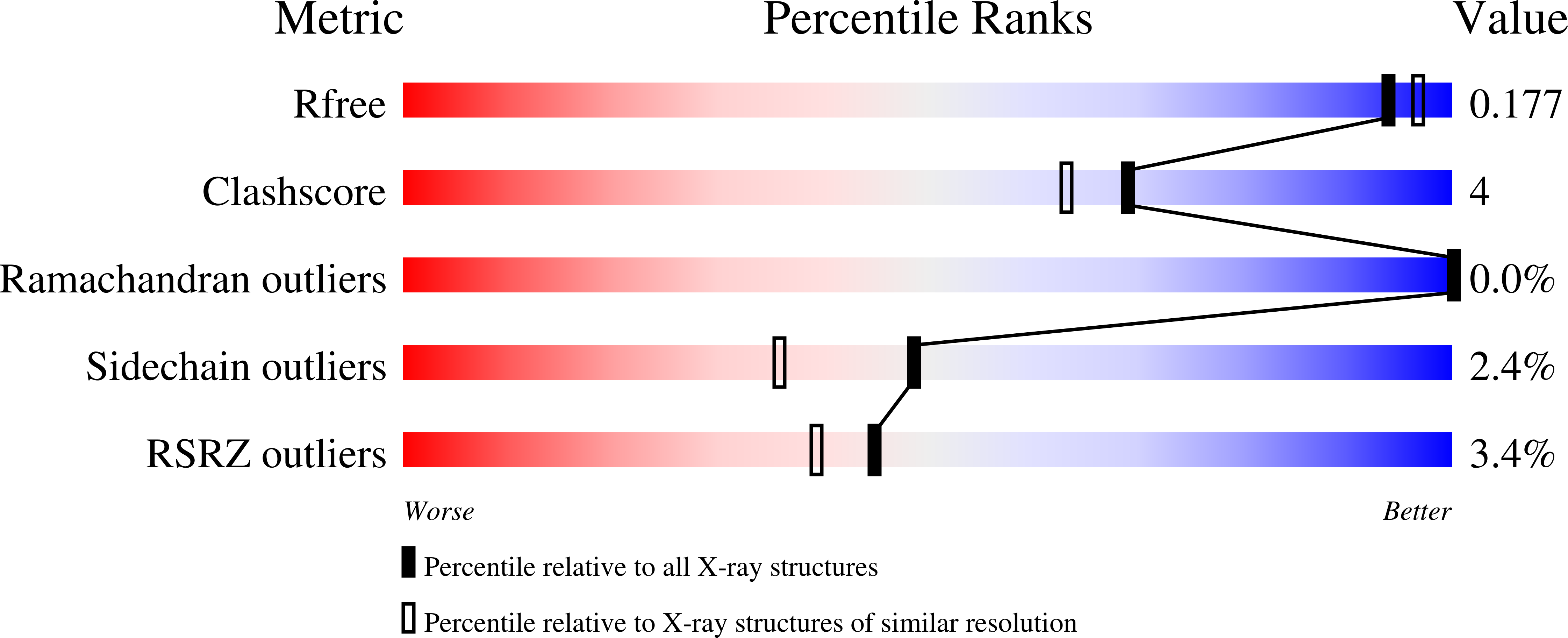
Pyranose 2-oxidase (P2Ox) is a 270 kDa homotetramer localized preferentially in the hyphal periplasmic space of lignocellulolytic fungi and has a proposed role in lignocellulose degradation to produce the essential co-substrate, hydrogen peroxide, for lignin peroxidases. P2Ox oxidizes D-glucose and other aldopyranoses regioselectively at C2 to the corresponding 2-keto sugars; however, for some substrates, the enzyme also displays specificity for oxidation at C3. The crystal structure of P2Ox from Trametes multicolor has been determined using single anomalous dispersion with mercury as anomalous scatterer. The model was refined at 1.8A resolution to R and Rfree values of 0.134 and 0.171, respectively. The overall fold of the P2Ox subunit resembles that of members of the glucose-methanol-choline family of long-chain oxidoreductases, featuring a flavin-binding Rossmann domain of class alpha/beta and a substrate-binding subdomain with a six-stranded central beta sheet and three alpha helices. The homotetramer buries a large internal cavity of roughly 15,000 A3, from which the four active sites are accessible. Four solvent channels lead from the surface into the cavity through which substrate must enter before accessing the active site. The present structure shows an acetate molecule bound in the active site with the carboxylate group positioned immediately below the flavin N5 atom, and with one carboxylate oxygen atom interacting with the catalytic residues His548 and Asn593. The entrance to the active site is blocked by a loop (residues 452 to 461) with excellent electron density but elevated temperature factors. We predict that this loop is dynamic and opens to allow substrate entry and exit. In silico docking of D-glucose in the P2Ox active site shows that with the active-site loop in the closed conformation, monosaccharides cannot be accommodated; however, after removing the loop from the model, a tentative set of protein-substrate interactions for beta-D-glucose have been outlined.
Organizational Affiliation:
Department of Biotechnology, KTH, Albanova University Center, SE-106 91 Stockholm, Sweden.