X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution.
Holden, H.M., Ito, M., Hartshorne, D.J., Rayment, I.(1992) J Mol Biol 227: 840-851
- PubMed: 1404391
- DOI: https://doi.org/10.1016/0022-2836(92)90226-a
- Primary Citation of Related Structures:
1FHG, 1TLK - PubMed Abstract:
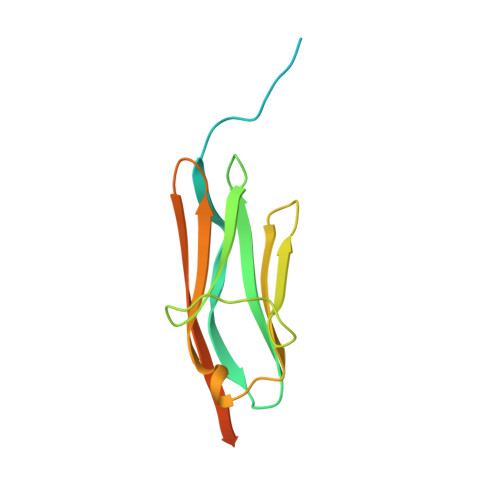
The three-dimensional structure of telokin, an acidic protein identical to the C-terminal portion of smooth muscle myosin light chain kinase from turkey gizzard, has been determined at 2.8 A resolution and refined to a crystallographic R-factor of 19.5% for all measured X-ray data from 30 A to 2.8 A. Crystals used in the investigation belonged to the space group P3(2)21, with one molecule per asymmetric unit and unit cell dimensions of a = b = 64.4 A and c = 50.6 A. Telokin contains 154 amino acid residues, 103 of which were visible in the electron density map. The overall molecular fold of telokin consists of seven strands of antiparallel beta-pleated sheet that wrap around to form a barrel. There is also an extended tail of eight amino acid residues at the N terminus that does not participate in beta-sheet formation. The beta-barrel can be simply envisioned as two layers of beta-sheet, nearly parallel to one another, with one layer containing four and the other three beta-strands. This type of beta-barrel, as seen in telokin, was first observed for the CH2 domain of an immunoglobulin fragment Fc. Telokin is an intracellular protein and, as such, does not contain the disulphide linkage between beta-strands B and F normally observed in the immunoglobulin constant domains. It does, however, contain two cysteine amino acid residues (Cys63 and Cys115) that are situated at structurally identical positions to those forming the disulphide linkage in the immunoglobulin constant domain.
Organizational Affiliation:
Institute for Enzyme Research, Graduate School, University of Wisconsin, Madison 53705.