The Peptide-Substrate-binding Domain of Collagen Prolyl 4-Hydroxylases Is a Tetratricopeptide Repeat Domain with Functional Aromatic Residues.
Pekkala, M., Hieta, R., Bergmann, U., Kivirikko, K.I., Wierenga, R.K., Myllyharju, J.(2004) J Biol Chem 279: 52255-52261
- PubMed: 15456751
- DOI: https://doi.org/10.1074/jbc.M410007200
- Primary Citation of Related Structures:
1TJC - PubMed Abstract:
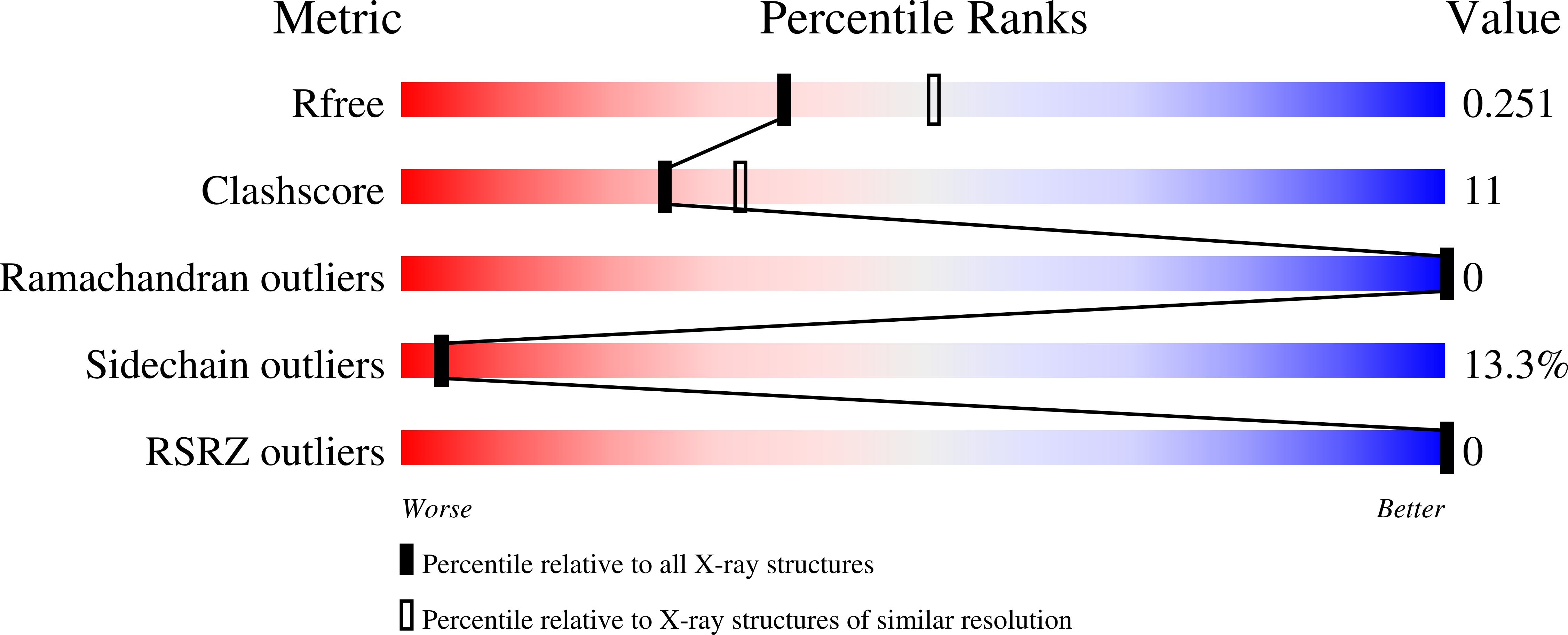
Collagen prolyl 4-hydroxylases catalyze the formation of 4-hydroxyproline in -X-Pro-Gly-sequences and have an essential role in collagen synthesis. The vertebrate enzymes are alpha2beta2 tetramers in which the catalytic alpha-subunits contain separate peptide-substrate-binding and catalytic domains. We report on the crystal structure of the peptide-substrate-binding domain of the human type I enzyme refined at 2.3 A resolution. It was found to belong to a family of tetratricopeptide repeat domains that are involved in many protein-protein interactions and consist of five alpha-helices forming two tetratricopeptide repeat motifs plus the solvating helix. A prominent feature of its concave surface is a deep groove lined by tyrosines, a putative binding site for proline-rich Tripeptides. Solvent-exposed side chains of three of the tyrosines have a repeat distance similar to that of a poly-L-proline type II helix. The aromatic surface ends at one of the tyrosines, where the groove curves almost 90 degrees away from the linear arrangement of the three tyrosine side chains, possibly inducing a bent conformation in the bound peptide. This finding is consistent with previous suggestions by others that a minimal structural requirement for proline 4-hydroxylation may be a sequence in the poly-L-proline type II conformation followed by a beta-turn in the Pro-Gly segment. Site-directed mutagenesis indicated that none of the tyrosines was critical for tetramer assembly, whereas most of them were critical for the binding of a peptide substrate and inhibitor both to the domain and the alpha2beta2 enzyme tetramer.
Organizational Affiliation:
Department of Biochemistry and Biocenter Oulu and Collagen Research Unit, University of Oulu, FIN-90014 Oulu, Finland.