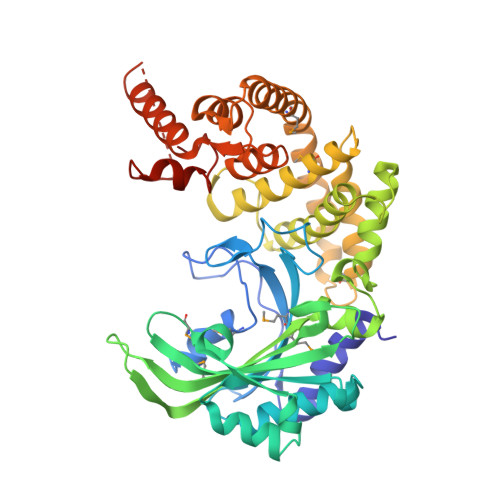
Alanyl-tRNA Synthetase Crystal Structure and Design for Acceptor-Stem Recognition
Swairjo, M.A., Otero, F.J., Yang, X.-L., Lovato, M.A., Skene, R.J., McRee, D.E., Ribas De Pouplana, L., Schimmel, P.(2004) Mol Cell 13: 829-841
- PubMed: 15053876
- DOI: https://doi.org/10.1016/s1097-2765(04)00126-1
- Primary Citation of Related Structures:
1RIQ - PubMed Abstract:
Early work on aminoacylation of alanine-specific tRNA (tRNA(Ala)) by alanyl-tRNA synthetase (AlaRS) gave rise to the concept of an early "second genetic code" imbedded in the acceptor stems of tRNAs. A single conserved and position-specific G:U base pair in the tRNA acceptor stem is the key identity determinant. Further understanding has been limited due to lack of a crystal structure of the enzyme. We determined a 2.14 A crystal structure of the 453 amino acid catalytic fragment of Aquifex aeolicus AlaRS. It contains the catalytic domain characteristic of class II synthetases, a helical domain with a hairpin motif critical for acceptor-stem recognition, and a C-terminal domain of a mixed alpha/beta fold. Docking of tRNA(Ala) on AlaRS shows critical contacts with the three domains, consistent with previous mutagenesis and functional data. It also suggests conformational flexibility within the C domain, which might allow for the positional variation of the key G:U base pair seen in some tRNA(Ala)s.
Organizational Affiliation:
Skaags Institute for Chemical Biology, Departments of Molecular Biology and Chemistry, The Scripps Research Institute, BCC-379, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.