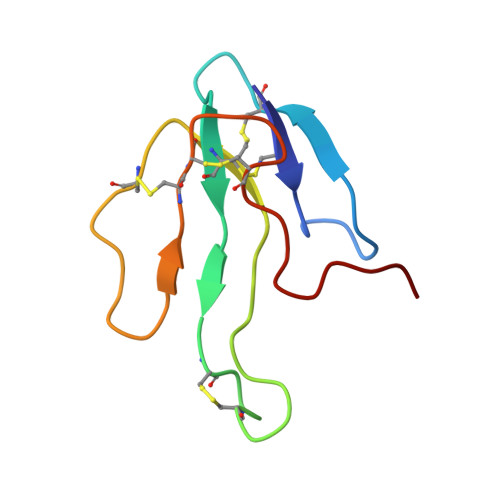
NMR and MD studies on the interaction between ligand peptides and alpha-bungarotoxin.
Bernini, A., Ciutti, A., Spiga, O., Scarselli, M., Klein, S., Vannetti, S., Bracci, L., Lozzi, L., Lelli, B., Falciani, C., Neri, P., Niccolai, N.(2004) J Mol Biol 339: 1169-1177
- PubMed: 15178256
- DOI: https://doi.org/10.1016/j.jmb.2004.04.041
- Primary Citation of Related Structures:
1RGJ - PubMed Abstract:
The interaction between alpha-bungarotoxin and linear synthetic peptides, mimotope of the nicotinic acetylcholine receptor binding site, has been characterised extensively by several methods and a wealth of functional, kinetic and structural data are available. Hence, this system represents a suitable model to explore in detail the dynamics of a peptide-protein interaction. Here, the solution structure of a new complex of the protein toxin with a tridecapeptide ligand exhibiting high affinity has been determined by NMR. As observed for three other previously reported mimotope-alpha-bungarotoxin complexes, also in this case correlations between biological activity and kinetic data are not fully consistent with a static discussion of structural data. Molecular dynamics simulations of the four mimotope-toxin complexes indicate that a relevant contribution to the complex stability is given by the extent of the residual flexibility that the protein maintains upon peptide binding. This feature, limiting the entropy loss caused by protein folding and binding, ought to be generally considered in a rational design of specific protein ligands.
Organizational Affiliation:
Biomolecular Structure Research Center and Department of Molecular Biology, University of Siena, via Fiorentina 1, I-53100 Siena, Italy. andrea.bernini@unisi.it