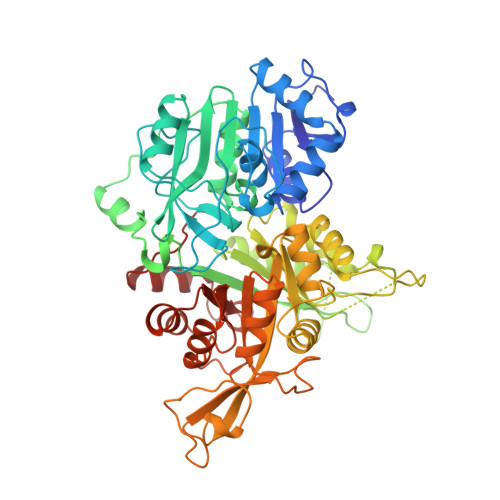
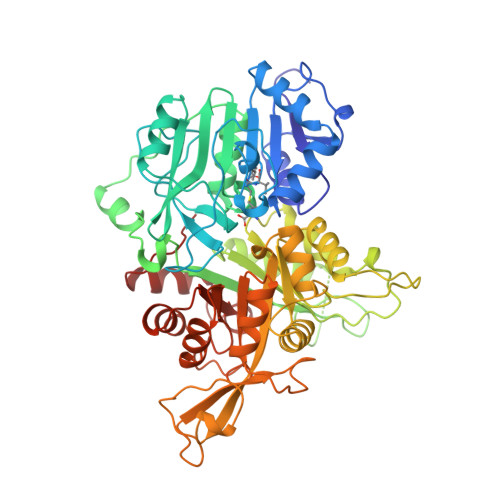
Towards Understanding the Mechanism of the Complex Cyclization Reaction Catalyzed by Imidazole Glycerophosphate Synthase: Crystal Structures of a Ternary Complex and the Free Enzyme
Chaudhuri, B.N., Lange, C., Myers, R.S., Davisson, V.J., Smith, J.L.(2003) Biochemistry 42: 7003-7012
- PubMed: 12795595
- DOI: https://doi.org/10.1021/bi034320h
- Primary Citation of Related Structures:
1OX4, 1OX5, 1OX6 - PubMed Abstract:
Imidazole glycerol phosphate synthase catalyzes formation of the imidazole ring in histidine biosynthesis. The enzyme is also a glutamine amidotransferase, which produces ammonia in a glutaminase active site and channels it through a 30-A internal tunnel to a cyclase active site. Glutaminase activity is impaired in the resting enzyme, and stimulated by substrate binding in the cyclase active site. The signaling mechanism was investigated in the crystal structure of a ternary complex in which the glutaminase active site was inactivated by a glutamine analogue and the unstable cyclase substrate was cryo-trapped in the active site. The orientation of N(1)-(5'-phosphoribulosyl)-formimino-5-aminoimidazole-4-carboxamide ribonucleotide in the cyclase active site implicates one side of the cyclase domain in signaling to the glutaminase domain. This side of the cyclase domain contains the interdomain hinge. Two interdomain hydrogen bonds, which do not exist in more open forms of the enzyme, are proposed as molecular signals. One hydrogen bond connects the cyclase domain to the substrate analogue in the glutaminase active site. The second hydrogen bond connects to a peptide that forms an oxyanion hole for stabilization of transient negative charge during glutamine hydrolysis. Peptide rearrangement induced by a fully closed domain interface is proposed to activate the glutaminase by unblocking the oxyanion hole. This interpretation is consistent with biochemical results [Myers, R. S., et al., (2003) Biochemistry 42, 7013-7022, the accompanying paper in this issue] and with structures of the free enzyme and a binary complex with a second glutamine analogue.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, Indiana 47907, USA.