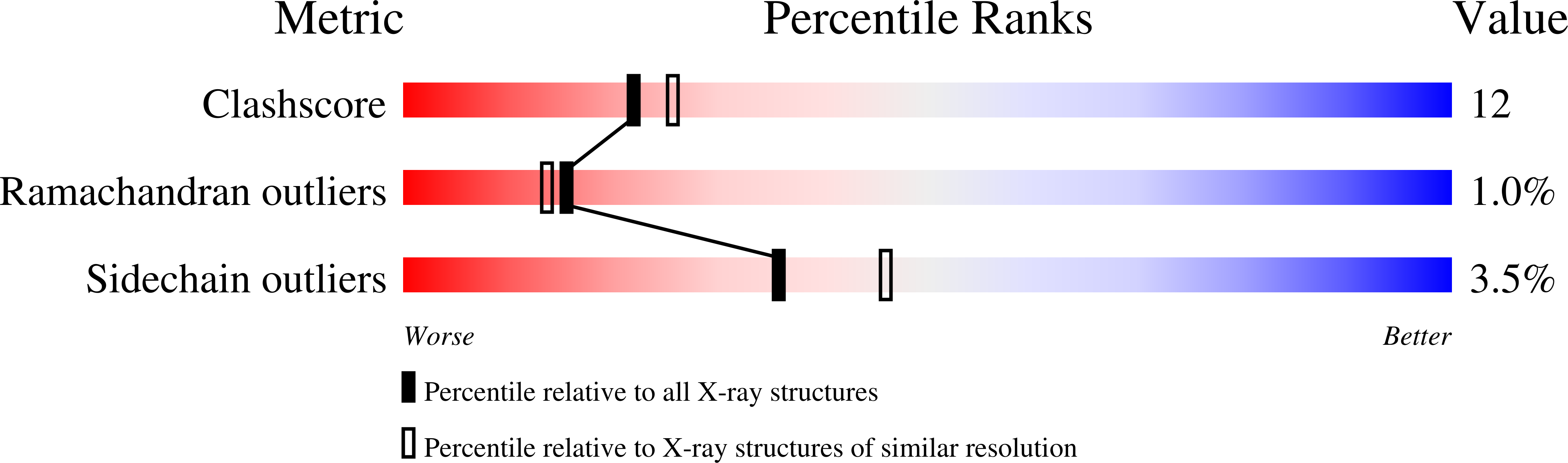Expanding pyrimidine diphosphosugar libraries via structure-based nucleotidylyltransferase engineering
Barton, W.A., Biggins, J.B., Jiang, J., Thorson, J.S., Nikolov, D.B.(2002) Proc Natl Acad Sci U S A 99: 13397-13402
- PubMed: 12374866
- DOI: https://doi.org/10.1073/pnas.192468299
- Primary Citation of Related Structures:
1MP3, 1MP4, 1MP5 - PubMed Abstract:
In vitro "glycorandomization" is a chemoenzymatic approach for generating diverse libraries of glycosylated biomolecules based on natural product scaffolds. This technology makes use of engineered variants of specific enzymes affecting metabolite glycosylation, particularly nucleotidylyltransferases and glycosyltransferases. To expand the repertoire of UDP/dTDP sugars readily available for glycorandomization, we now report a structure-based engineering approach to increase the diversity of alpha-d-hexopyranosyl phosphates accepted by Salmonella enterica LT2 alpha-d-glucopyranosyl phosphate thymidylyltransferase (E(p)). This article highlights the design rationale, determined substrate specificity, and structural elucidation of three "designed" mutations, illustrating both the success and unexpected outcomes from this type of approach. In addition, a single amino acid substitution in the substrate-binding pocket (L89T) was found to significantly increase the set of alpha-d-hexopyranosyl phosphates accepted by E(p) to include alpha-d-allo-, alpha-d-altro-, and alpha-d-talopyranosyl phosphate. In aggregate, our results provide valuable blueprints for altering nucleotidylyltransferase specificity by design, which is the first step toward in vitro glycorandomization.
Organizational Affiliation:
Cellular Biochemistry and Biophysics Program, Memorial Sloan-Kettering Cancer Center, New York, NY 10021, USA.