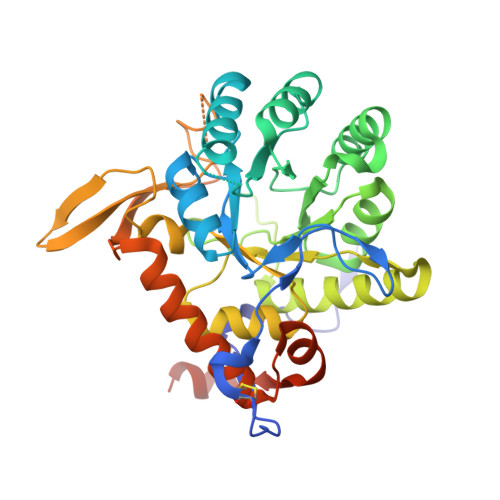
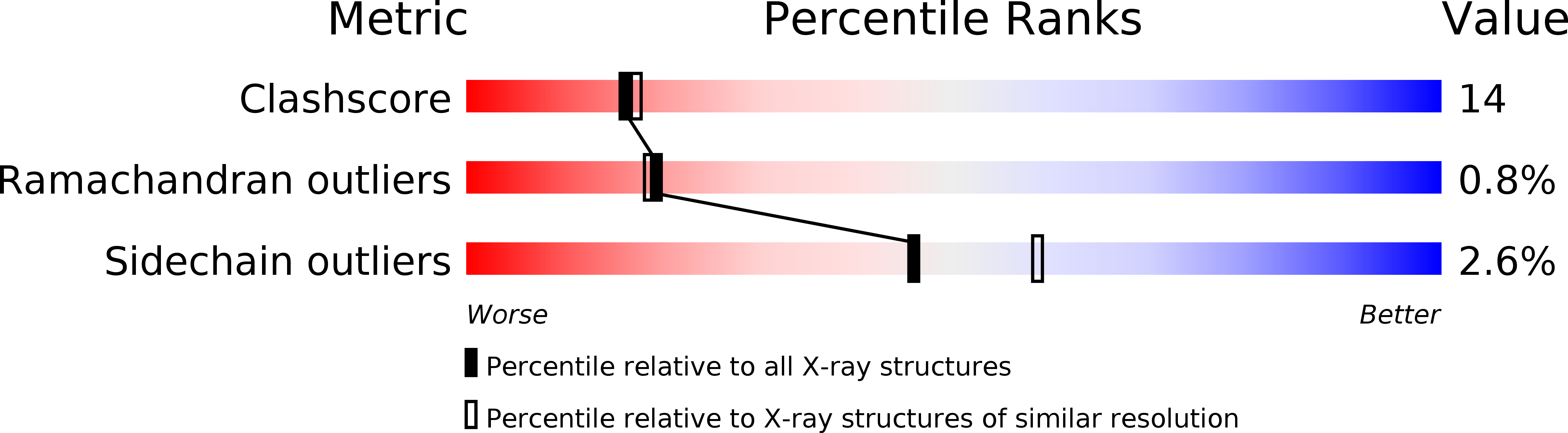Crystal structure of a ternary complex of Tritrichomonas foetus inosine 5'-monophosphate dehydrogenase: NAD+ orients the active site loop for catalysis
Gan, L., Petsko, G.A., Hedstrom, L.(2003) Biochemistry 41: 13309-13317
- PubMed: 12403633
- DOI: https://doi.org/10.1021/bi0203785
- Primary Citation of Related Structures:
1LRT - PubMed Abstract:
Inosine 5'-monophosphate dehydrogenase (IMPDH) catalyzes the conversion of IMP to XMP with the reduction of NAD(+), which is the rate-limiting step in the biosynthesis of guanine nucleotides. IMPDH is a promising target for chemotherapy. Microbial IMPDHs differ from mammalian enzymes in their lower affinity for inhibitors such as mycophenolic acid (MPA) and thiazole-4-carboxamide adenine dinucleotide (TAD). Part of this resistance is determined by the coupling between nicotinamide and adenosine subsites in the NAD(+) binding site that is postulated to involve an active site flap. To understand the structural basis of the drug selectivity, we solved the X-ray crystal structure of the catalytic core domain of Tritrichomonas foetus IMPDH in complex with IMP and beta-methylene-TAD at 2.2 A resolution. Unlike previous structures of this enzyme, the active site loop is ordered in this complex, and the catalytic Cys319 is 3.6 A from IMP, in the same plane as the hypoxanthine ring. The active site loop forms hydrogen bonds to the carboxamide of beta-Me-TAD which suggests that NAD(+) promotes the nucleophillic attack of Cys319 on IMP. The interactions of the adenosine end of TAD are very different from those in the human enzyme, suggesting the NAD(+) site may be an exploitable target for the design of antimicrobial drugs. In addition, a new K(+) site is observed at the subunit interface. This site is adjacent to beta-Me-TAD, consistent with the link between the K(+) activation and NAD(+). However, contrary to the coupling model, the flap does not cover the adenosine subsite and remains largely disordered.
Organizational Affiliation:
Department of Biochemistry, Brandeis University, Waltham, Massachusetts 02454, USA.