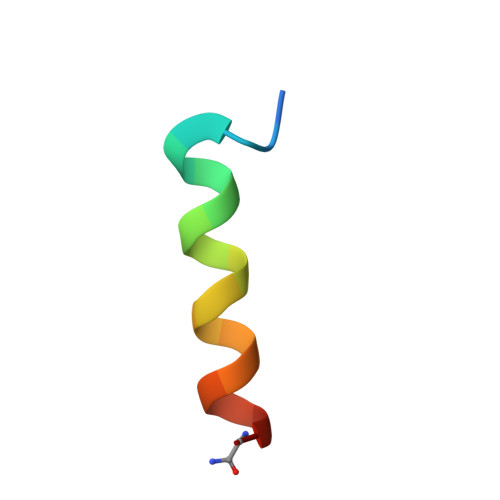
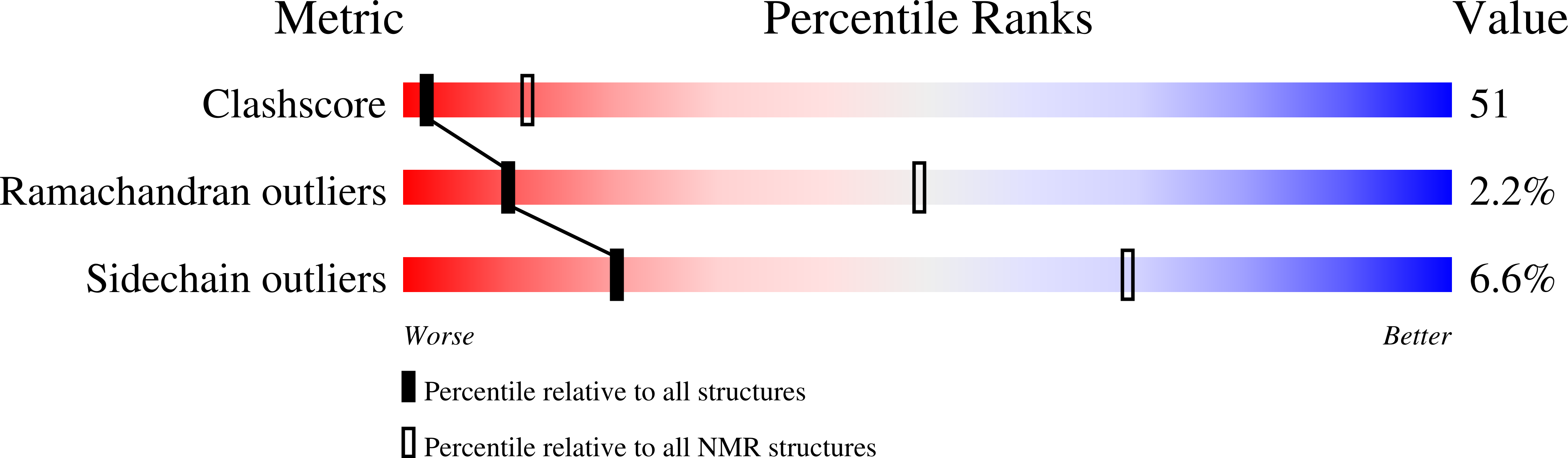Structure of human immunodeficiency virus type 1 Vpr(34-51) peptide in micelle containing aqueous solution.
Engler, A., Stangler, T., Willbold, D.(2002) Eur J Biochem 269: 3264-3269
- PubMed: 12084067
- DOI: https://doi.org/10.1046/j.1432-1033.2002.03005.x
- Primary Citation of Related Structures:
1KZS, 1KZT, 1KZV - PubMed Abstract:
Human immunodeficiency virus type 1 protein R (HIV-1 Vpr) promotes nuclear entry of viral nucleic acids in nondividing cells, causes G(2) cell cycle arrest and is involved in cellular differentiation and cell death. Vpr subcellular localization is as variable as its functions. It is known, that consistent with its role in nuclear transport, Vpr localizes to the nuclear envelope of human cells. Further, a reported ion channel activity of Vpr is clearly dependent on its localization in or at membranes. We focused our structural studies on the secondary structure of a peptide consisting of residues 34-51 of HIV-1 Vpr. This part of Vpr plays an important role in Vpr oligomerization, contributes to cell cycle arrest activity, and is essential for virion incorporation and binding to HHR23A, a protein involved in DNA repair. Employing NMR spectroscopy we found this part of Vpr to be almost completely alpha helical in the presence of micelles, as well as in trifluoroethanol containing and methanol/chloroform solvent. Our results provide structural data suggesting residues 34-51 of Vpr to contain an amphipathic, leucine-zipper-like alpha helix, which serves as a basis for oligomerization of Vpr and its interactions with cellular and viral factors involved in subcellular localization and virion incorporation of Vpr.
Organizational Affiliation:
Lehrstuhl für Biopolymere, Universität Bayreuth, Germany.