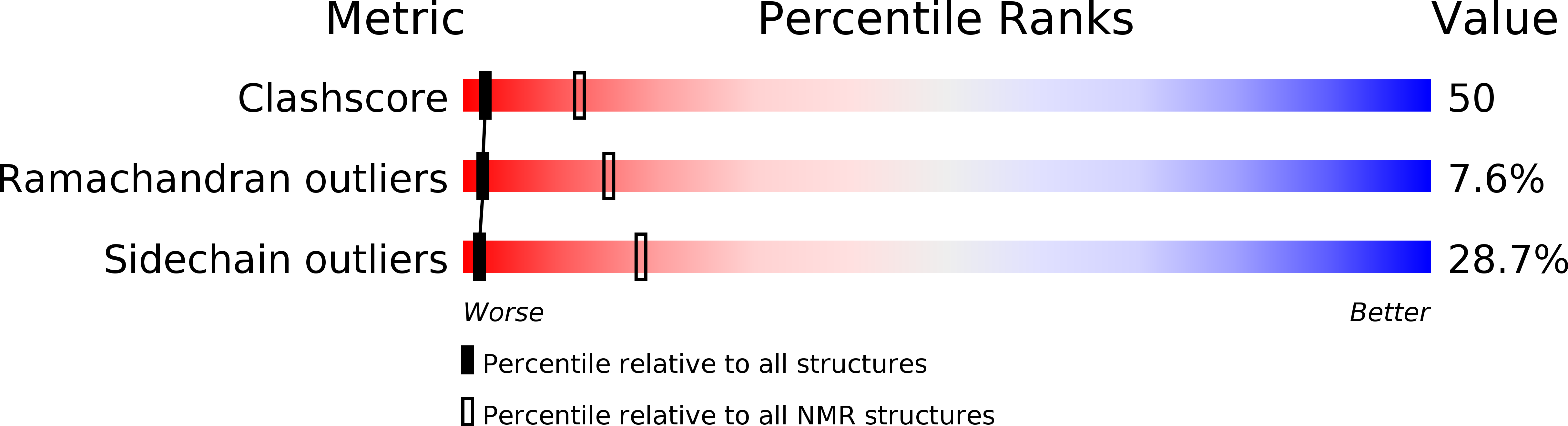Structure of a paralytic peptide from an insect, Manduca sexta.
Yu, X.Q., Prakash, O., Kanost, M.R.(1999) J Pept Res 54: 256-261
- PubMed: 10517164
- DOI: https://doi.org/10.1034/j.1399-3011.1999.00136.x
- Primary Citation of Related Structures:
1HRL - PubMed Abstract:
Paralytic peptide 1 (PP1) from a moth, Manduca sexta, is a 23-residue peptide (Glu-Asn-Phe-Ala-Gly-Gly-Cys-Ala-Thr-Gly-Tyr-Leu-Arg-Thr-Ala-Asp-Gly-Arg -Cys-Lys-Pro-Thr-Phe) that was first found to have paralytic activity when injected into M. sexta larvae. Recent studies demonstrated that PP1 also stimulated the spreading and aggregation of a blood cell type called plasmatocytes and inhibited bleeding from wounds. We determined the solution structure of PP1 by two-dimensional 1H NMR spectroscopy to begin to understand structural-functional relationships of this peptide. PP1 has an ordered structure, which is composed of a short antiparallel beta-sheet at residues Tyr11-Thr14 and Arg18-Pro21, three beta turns at residues Phe3-Gly6, Ala8-Tyr11 and Thr14-Gly17, and a half turn at the carboxyl-terminus (residues Lys20-Phe23). The well-defined secondary and tertiary structure was stabilized by hydrogen bonding and side-chain hydrophobic interactions. In comparison with two related insect peptides, whose structures have been solved recently, the amino-terminal region of PP1 is substantially more ordered. The short antiparallel beta-sheet of PP1 has a folding pattern similar to the carboxyl-terminal subdomain of epidermal growth factor (EGF). Therefore, PP1 may interact with EGF receptor-like molecules to trigger its different biological activities.
Organizational Affiliation:
Department of Biochemistry, Kansas State University, Manhattan 66506, USA.