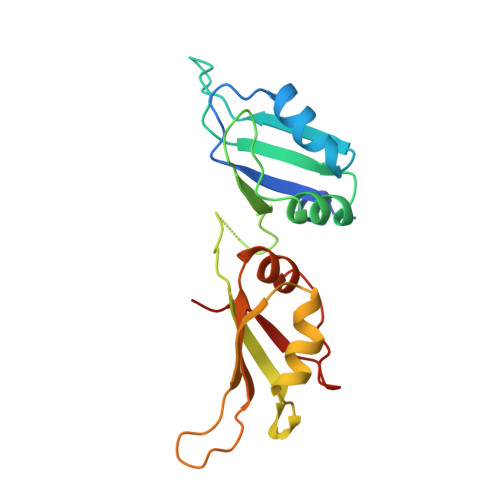
Crystal structure of the two RNA binding domains of human hnRNP A1 at 1.75 A resolution.
Shamoo, Y., Krueger, U., Rice, L.M., Williams, K.R., Steitz, T.A.(1997) Nat Struct Biol 4: 215-222
- PubMed: 9164463
- DOI: https://doi.org/10.1038/nsb0397-215
- Primary Citation of Related Structures:
1HA1 - PubMed Abstract:
Heterogeneous ribonucleoprotein A1 (hnRNP A1) is an abundant eukaryotic nuclear RNA binding protein. A1 is involved in the packaging of pre-mRNA into hnRNP particles, transport of poly A+ mRNA from the nucleus to the cytoplasm and may modulate splice site selection. The crystal structure of A1(RBD1,2) reveals two independently-folded RNA binding domains (RBDs) connected by a flexible linker. Both RBDs are structurally homologous to the U1A(RBD1), and have their RNA binding platforms oriented in an anti-parallel fashion. The anti-parallel arrangement of the A1 RNA binding platforms suggests mechanisms for RNA condensation and ways of bringing together distant RNA sequences for RNA metabolism such as splicing or transport.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520-8114, USA.