Specificity determinants in inositol polyphosphate synthesis: crystal structure of inositol 1,3,4-trisphosphate 5/6-kinase.
Miller, G.J., Wilson, M.P., Majerus, P.W., Hurley, J.H.(2005) Mol Cell 18: 201-212
- PubMed: 15837423
- DOI: https://doi.org/10.1016/j.molcel.2005.03.016
- Primary Citation of Related Structures:
1Z2N, 1Z2O, 1Z2P - PubMed Abstract:
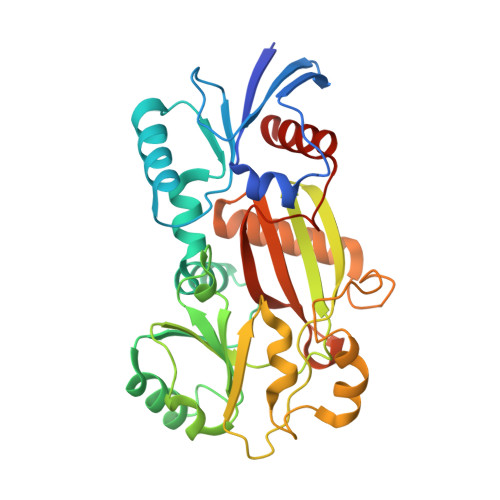
Inositol hexakisphosphate and other inositol high polyphosphates have diverse and critical roles in eukaryotic regulatory pathways. Inositol 1,3,4-trisphosphate 5/6-kinase catalyzes the rate-limiting step in inositol high polyphosphate synthesis in animals. This multifunctional enzyme also has inositol 3,4,5,6-tetrakisphosphate 1-kinase and other activities. The structure of an archetypal family member, from Entamoeba histolytica, has been determined to 1.2 A resolution in binary and ternary complexes with nucleotide, substrate, and product. The structure reveals an ATP-grasp fold. The inositol ring faces ATP edge-on such that the 5- and 6-hydroxyl groups are nearly equidistant from the ATP gamma-phosphate in catalytically productive phosphoacceptor positions and explains the unusual dual site specificity of this kinase. Inositol tris- and tetrakisphosphates interact via three phosphate binding subsites and one solvent-exposed site that could in principle be occupied by 18 different substrates, explaining the mechanisms for the multiple specificities and catalytic activities of this enzyme.
Organizational Affiliation:
Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, United States Department of Health and Human Services, Bethesda, Maryland 20892, USA.