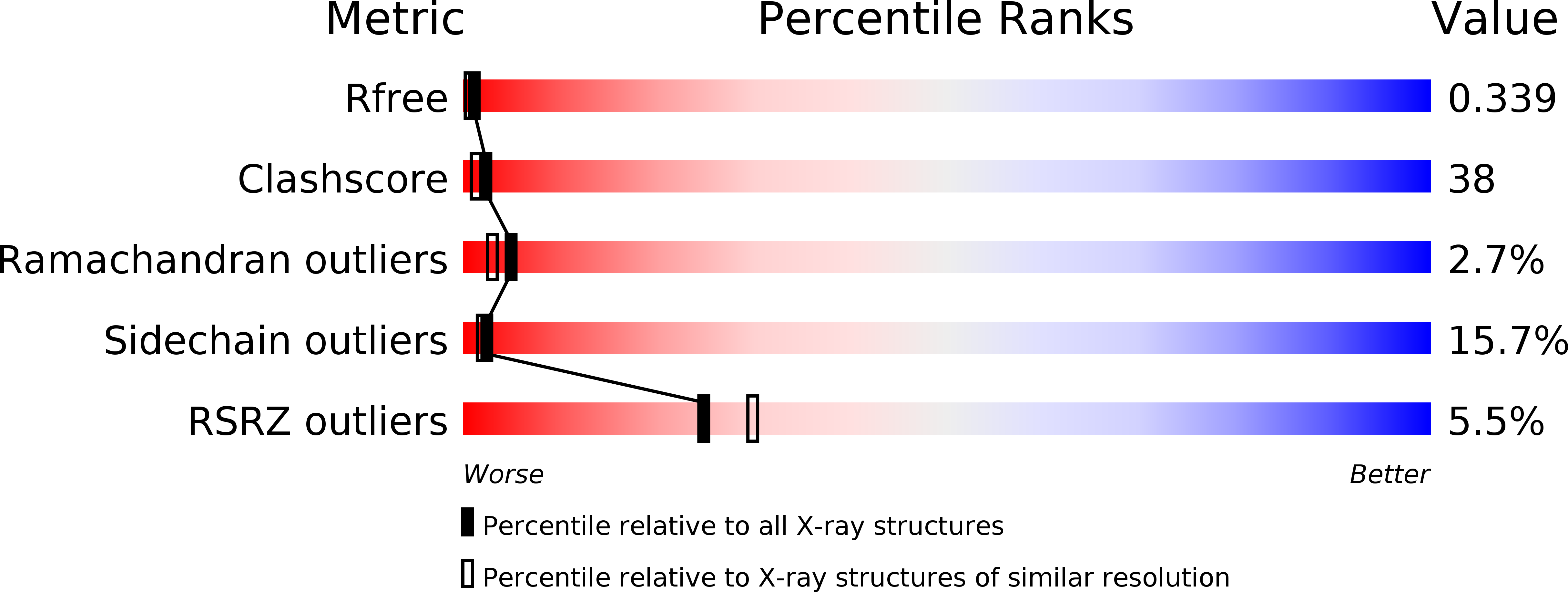
Atomic-resolution Crystal Structure of the Proteolytic Domain of Archaeoglobus fulgidus Lon Reveals the Conformational Variability in the Active Sites of Lon Proteases
Botos, I., Melnikov, E.E., Cherry, S., Kozlov, S., Makhovskaya, O.V., Tropea, J.E., Gustchina, A., Rotanova, T.V., Wlodawer, A.(2005) J Mol Biol 351: 144-157
- PubMed: 16002085
- DOI: https://doi.org/10.1016/j.jmb.2005.06.008
- Primary Citation of Related Structures:
1Z0B, 1Z0C, 1Z0E, 1Z0G, 1Z0W - PubMed Abstract:
The atomic-resolution crystal structure of the proteolytic domain (P-domain, residues 415-621) of Archaeoglobus fulgidus B-type Lon protease (wtAfLonB) and the structures of several mutants have revealed significant differences in the conformation of the active-site residues when compared to other known Lon P-domains, despite the conservation of the overall fold. The catalytic Ser509 is facing the solvent and is distant from Lys552, the other member of the catalytic dyad. Instead, the adjacent Asp508 forms an ion pair with the catalytic lysine residue. Glu506, an analog of the putative third catalytic residue from a related Methanococcus jannaschii LonB, also faces the solvent and does not interact with the catalytic dyad. We have established that full-length wtAfLonB is proteolytically active in an ATP-dependent manner. The loss of enzymatic activity of the S509A mutant confirms the functional significance of this residue, while retention of considerable level of activity by the D508A and E506A mutants rules out their critical involvement in catalysis. In contrast to the full-length enzymes, all individually purified P-domains (wild-type and mutants) were inactive, and the mutations had no influence on the active-site structure. These findings raise the possibility that, although isolated proteolytic domains of both AfLonB and E.coli LonA are able to assemble into expected functional hexamers, the presence of the other domains, as well as substrate binding, may be needed to stabilize the productive conformation of their active sites. Thus, the observed conformational variability may reflect the differences in the stability of active-site structures for the proteolytic counterparts of single-chain Lon versus independently folded proteolytic subunits of two-chain AAA+ proteases.
Organizational Affiliation:
Macromolecular Crystallography Laboratory, National Cancer Institute at Frederick, Frederick, MD 21702-1201, USA.