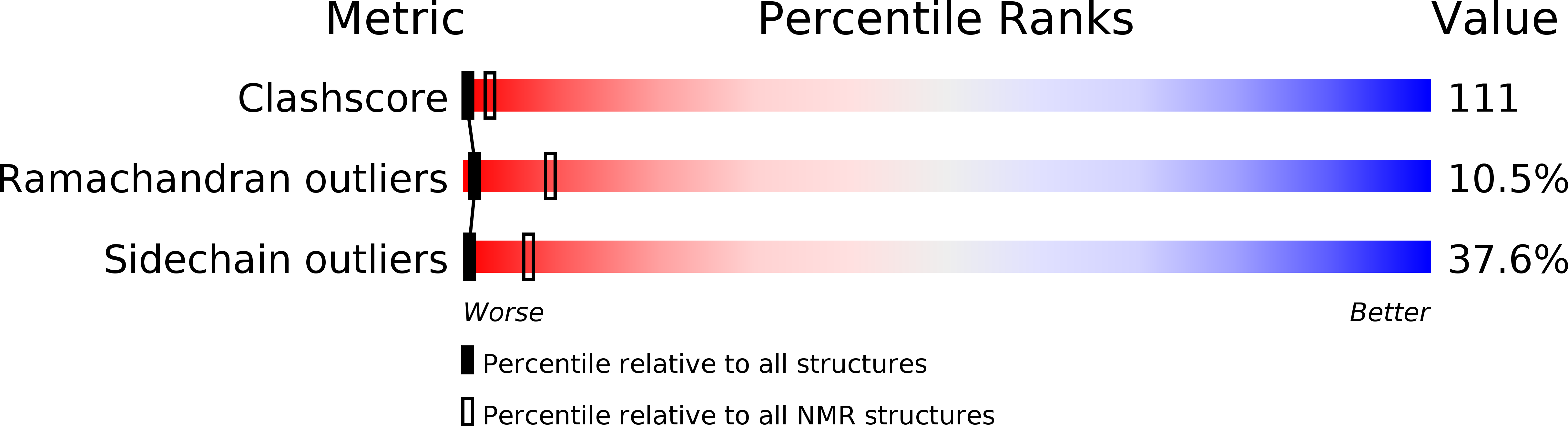NMR studies of a viral protein that mimics the regulators of complement activation.
Wiles, A.P., Shaw, G., Bright, J., Perczel, A., Campbell, I.D., Barlow, P.N.(1997) J Mol Biol 272: 253-265
- PubMed: 9299352
- DOI: https://doi.org/10.1006/jmbi.1997.1241
- Primary Citation of Related Structures:
1VVC, 1VVD, 1VVE - PubMed Abstract:
Vaccinia virus complement control protein (VCP) is a 243-residue protein that is similar in sequence to the regulators of complement activation; its role is to defend the virus against attack by the host complement system. A fragment of this protein spanning the two complement protein (CP)-modules (residues 126 to 243) which make up the C-terminal half of VCP has been expressed in Pichia pastoris. A 15N-labelled sample was purified for the purposes of structure determination and measurements of dynamics in solution using NMR. Structures were calculated on the basis of 1767 NMR-derived distance and angle restraints, with a longer than normal high-temperature simulated annealing (SA) protocol which improved convergence. The viral CP-modules are structurally very similar to the 15th and 16th CP-modules of human factor H (fH; average r.m.s.d., for invariant Trp and Cys, four pair-wise comparisons,=1.2 A) but less similar to the fifth CP-module of fH (average r.m.s.d.=2.2 A). In the VCP fragment, the orientation of one module with respect to the other is clearly defined by the experimental data, and T1 measurements are consistent with only limited flexibility at the module-module interface. The r.m.s.d. over all of the 118 residues (backbone atoms) is 0.73 A. The intermodular orientation is better defined than, and significantly different from, that observed in a CP-module pair from fH (re-calculated using the extended SA protocol). In VCP the long axis of the second module is tilted by 59(+/-4) degrees with respect to the first module (50(+/-13) degrees in the fH pair), and twisted with respect to the first module by 22(+/-6) degrees (223(+/-17) degrees in fH). The differences between the human and viral proteins may be rationalised in terms of the lack of hydrogen-bond stabilised secondary structure in the N-terminal portion of fH module 16, and the number and type of amino acid side-chains which make up the interface. A similar intermodular interface may be predicted between the third and fourth module of human C4 binding protein and, probably, between the third and fourth modules of the guinea pig acrosomal matrix protein 67; but the formulation of general rules for predicting the structure of interfaces between CP-modules awaits further experimental data.
Organizational Affiliation:
The Edinburgh Centre for Protein Technology, The University of Edinburgh The James Black Building The King's Buildings, West Mains Road, Edinburgh, EH9 3JJ, UK.