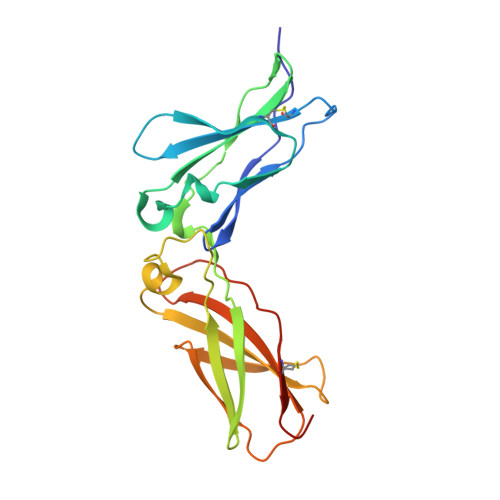
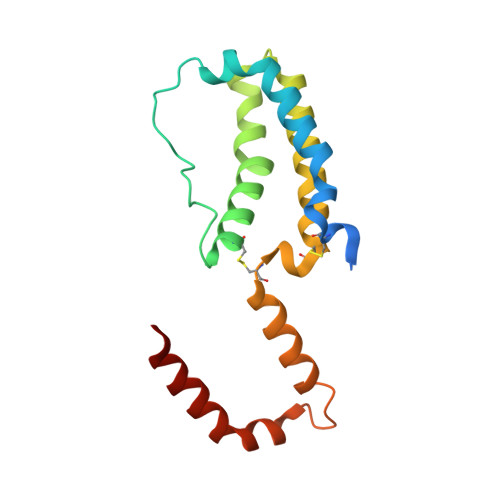
Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1.
Jones, B.C., Logsdon, N.J., Josephson, K., Cook, J., Barry, P.A., Walter, M.R.(2002) Proc Natl Acad Sci U S A 99: 9404-9409
- PubMed: 12093920
- DOI: https://doi.org/10.1073/pnas.152147499
- Primary Citation of Related Structures:
1LQS - PubMed Abstract:
Human IL-10 (hIL-10) modulates critical immune and inflammatory responses by way of interactions with its high- (IL-10R1) and low-affinity (IL-10R2) cell surface receptors. Human cytomegalovirus exploits the IL-10 signaling pathway by expressing a functional viral IL-10 homolog (cmvIL-10), which shares only 27% sequence identity with hIL-10 yet signals through IL-10R1 and IL-10R2. To define the molecular basis of this virus-host interaction, we determined the 2.7-A crystal structure of cmvIL-10 bound to the extracellular fragment of IL-10R1 (sIL-10R1). The structure reveals cmvIL-10 forms a disulfide-linked homodimer that binds two sIL-10R1 molecules. Although cmvIL-10 and hIL-10 share similar intertwined topologies and sIL-10R1 binding sites, their respective interdomain angles differ by approximately 40 degrees. This difference results in a striking re-organization of the IL-10R1s in the putative cell surface complex. Solution binding studies show cmvIL-10 and hIL-10 share essentially identical affinities for sIL-10R1 whereas the Epstein-Barr virus IL-10 homolog (ebvIL-10), whose structure is highly similar to hIL-10, exhibits a approximately 20-fold reduction in sIL-10R1 affinity. Our results suggest cmvIL-10 and ebvIL-10 have evolved different molecular mechanisms to engage the IL-10 receptors that ultimately enhance the respective ability of their virus to escape immune detection.
Organizational Affiliation:
Center for Biophysical Sciences and Engineering, Department of Microbiology, University of Alabama, 1025 18th Street South, Birmingham, AL 35294, USA.