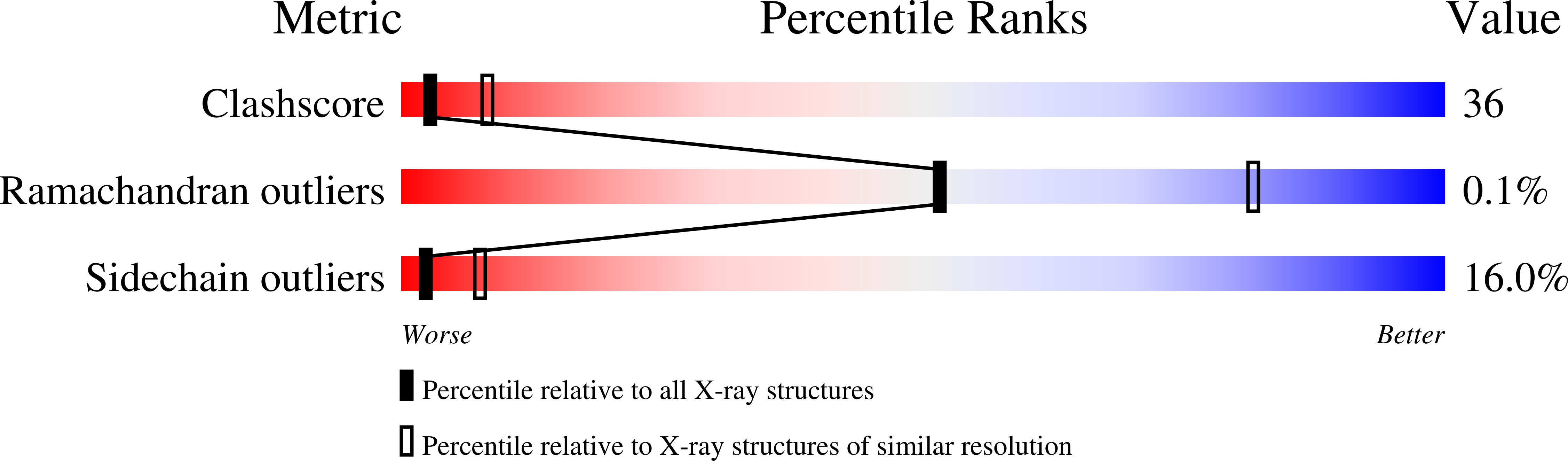Catalytic Mechanism of S-adenosylhomocysteine hydrolase. Site-directed mutagenesis of Asp-130, Lys-185, Asp-189, and Asn-190.
Takata, Y., Yamada, T., Huang, Y., Komoto, J., Gomi, T., Ogawa, H., Fujioka, M., Takusagawa, F.(2002) J Biol Chem 277: 22670-22676
- PubMed: 11927587
- DOI: https://doi.org/10.1074/jbc.M201116200
- Primary Citation of Related Structures:
1KY4, 1KY5 - PubMed Abstract:
S-Adenosylhomocysteine hydrolase (AdoHcyase) catalyzes the hydrolysis of S-adenosylhomocysteine to form adenosine and homocysteine. On the bases of crystal structures of the wild type enzyme and the D244E mutated enzyme complexed with 3'-keto-adenosine (D244E.Ado*), we have identified the important amino acid residues, Asp-130, Lys-185, Asp-189, and Asn-190, for the catalytic reaction and have proposed a catalytic mechanism (Komoto, J., Huang, Y., Gomi, T., Ogawa, H., Takata, Y., Fujioka, M., and Takusagawa, F. (2000) J. Biol. Chem. 275, 32147-32156). To confirm the proposed catalytic mechanism, we have made the D130N, K185N, D189N, and N190S mutated enzymes and measured the catalytic activities. The catalytic rates (k(cat)) of D130N, K185N, D189N, and N190S mutated enzymes are reduced to 0.7%, 0.5%, 0.1%, and 0.5%, respectively, in comparison with the wild type enzyme, indicating that Asp-130, Lys-185, Asp-189, and Asn-190 are involved in the catalytic reaction. K(m) values of the mutated enzymes are increased significantly, except for the N190S mutation, suggesting that Asp-130, Lys-185, and Asp-189 participate in the substrate binding. To interpret the kinetic data, the oxidation states of the bound NAD molecules of the wild type and mutated enzymes were measured during the catalytic reaction by monitoring the absorbance at 340 nm. The crystal structures of the WT and D244E.Ado*, containing four subunits in the crystallographic asymmetric unit, were re-refined to have the same subunit structures. A detailed catalytic mechanism of AdoHcyase has been revealed based on the oxidation states of the bound NAD and the re-refined crystal structures of WT and D244E.Ado*. Lys-185 and Asp-130 abstract hydrogen atoms from 3'-OH and 4'-CH, respectively. Asp-189 removes a proton from Lys-185 and produces the neutral N zeta (-NH(2)), and Asn-190 facilitates formation of the neutral Lys-185. His-54 and His-300 hold and polarize a water molecule, which nucleophilically attacks the C5'- of 3'-keto-4',5'-dehydroadenosine to produce 3'-keto-Ado.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, Kansas 66045-7534, USA.