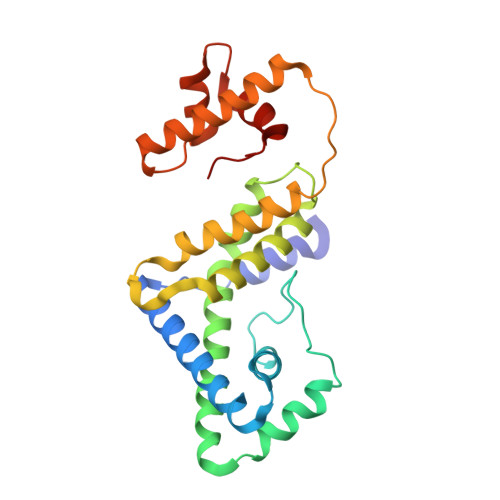
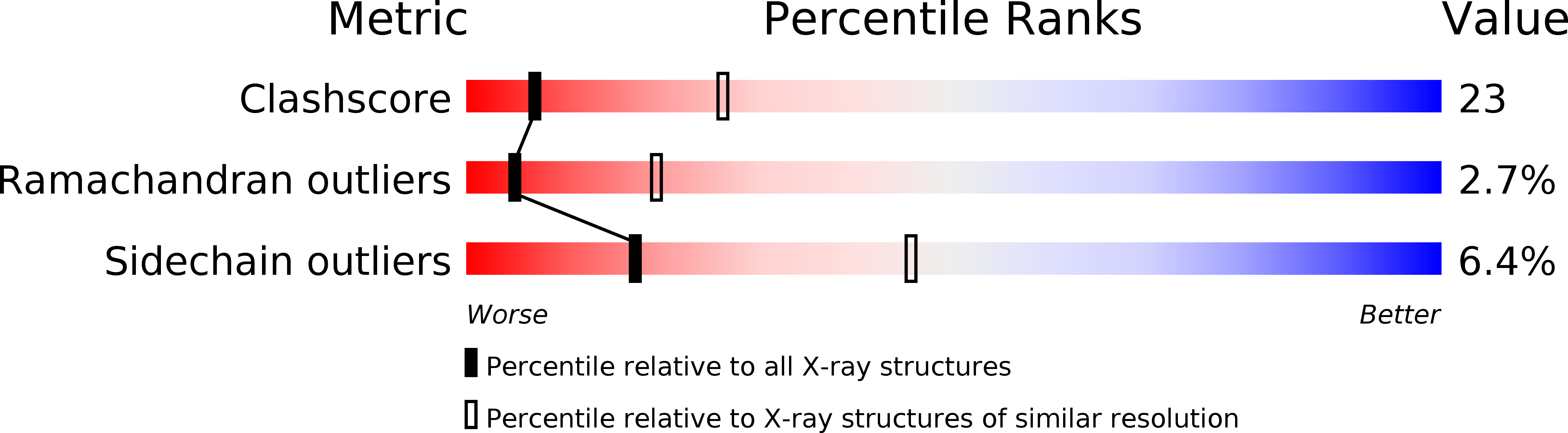Structure of the bacterial RNA polymerase promoter specificity sigma subunit.
Campbell, E.A., Muzzin, O., Chlenov, M., Sun, J.L., Olson, C.A., Weinman, O., Trester-Zedlitz, M.L., Darst, S.A.(2002) Mol Cell 9: 527-539
- PubMed: 11931761
- DOI: https://doi.org/10.1016/s1097-2765(02)00470-7
- Primary Citation of Related Structures:
1KU2, 1KU3, 1KU7 - PubMed Abstract:
The sigma subunit is the key regulator of bacterial transcription. Proteolysis of Thermus aquaticus sigma(A), which occurred in situ during crystallization, reveals three domains, sigma(2), sigma(3), and sigma(4), connected by flexible linkers. Crystal structures of each domain were determined, as well as of sigma(4) complexed with -35 element DNA. Exposed surfaces of each domain are important for RNA polymerase binding. Universally conserved residues important for -10 element recognition and melting lie on one face of sigma(2), while residues important for extended -10 recognition lie on sigma(3). Genetic studies correctly predicted that a helix-turn-helix motif in sigma(4) recognizes the -35 element but not the details of the protein-DNA interactions. Positive control mutants in sigma(4) cluster in two regions, positioned to interact with activators bound just upstream or downstream of the -35 element.
Organizational Affiliation:
Laboratory of Molecular Biophysics, The Rockefeller University, 1230 York Avenue, New York, NY 10021, USA.