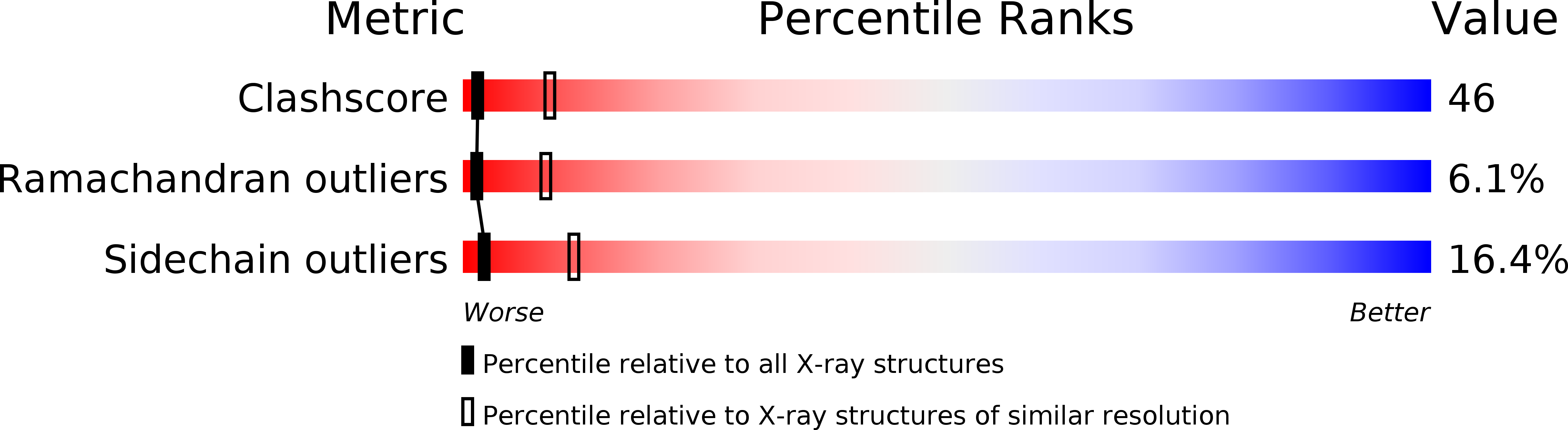Peptide exosite inhibitors of factor VIIa as anticoagulants.
Dennis, M.S., Eigenbrot, C., Skelton, N.J., Ultsch, M.H., Santell, L., Dwyer, M.A., O'Connell, M.P., Lazarus, R.A.(2000) Nature 404: 465-470
- PubMed: 10761907
- DOI: https://doi.org/10.1038/35006574
- Primary Citation of Related Structures:
1DVA - PubMed Abstract:
Potent anticoagulants have been derived by targeting the tissue factor-factor VIIa complex with naive peptide libraries displayed on M13 phage. The peptides specifically block the activation of factor X with a median inhibitory concentration of 1 nM and selectively inhibit tissue-factor-dependent clotting. The peptides do not bind to the active site of factor VIIa; rather, they work by binding to an exosite on the factor VIIa protease domain, and non-competitively inhibit activation of factor X and amidolytic activity. One such peptide (E-76) has a well defined structure in solution determined by NMR spectroscopy that is similar to the X-ray crystal structure when complexed with factor VIIa. These structural and functional studies indicate an allosteric 'switch' mechanism of inhibition involving an activation loop of factor VIIa and represent a new framework for developing inhibitors of serine proteases.
Organizational Affiliation:
Department of Protein Engineering, Genentech, Inc., South San Francisco, California 94080, USA.