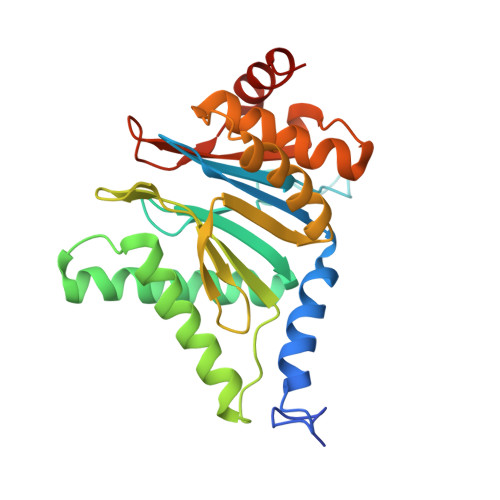
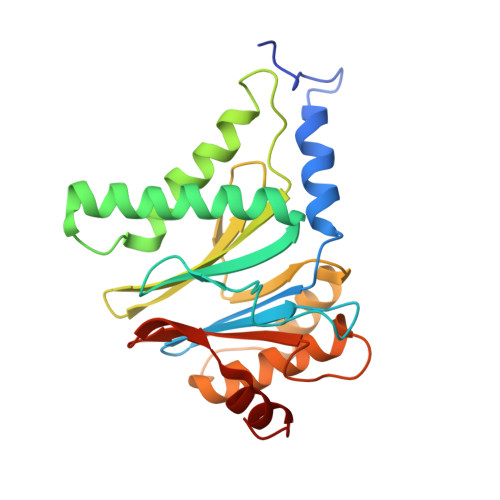
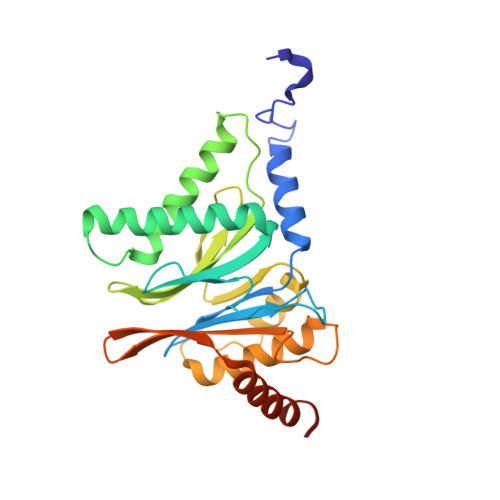
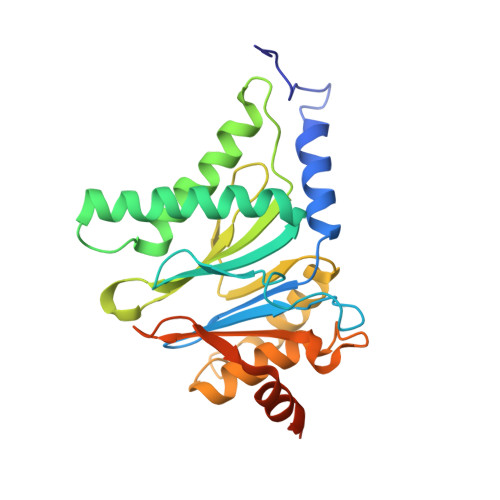
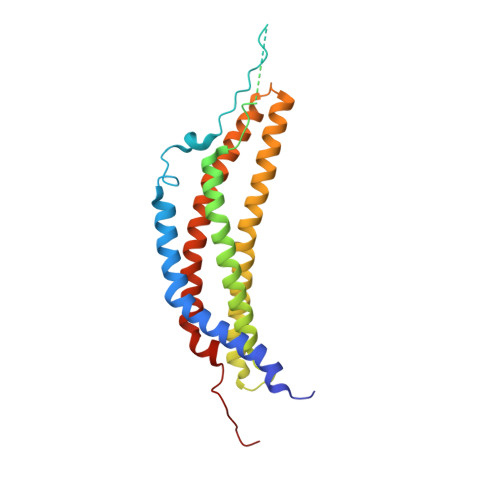
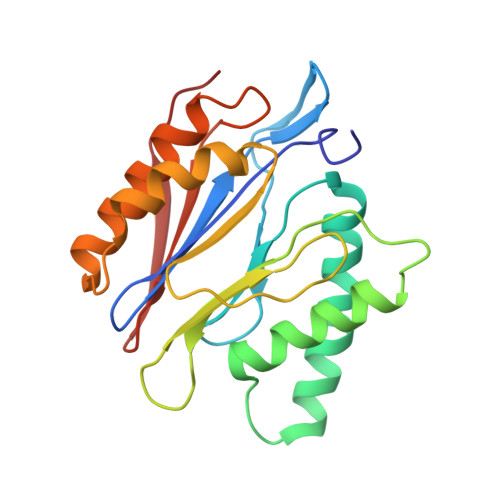
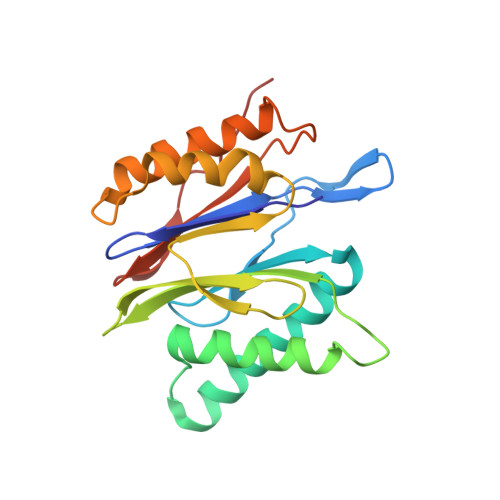
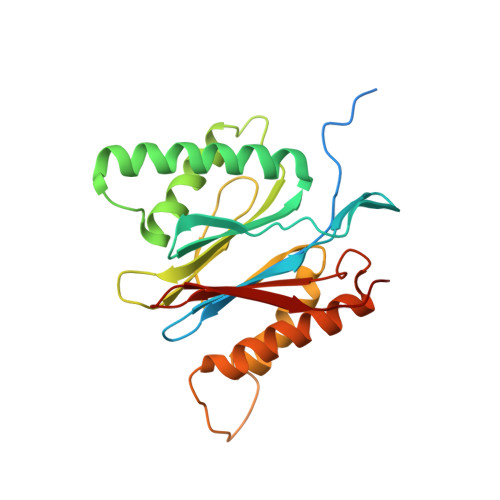
Cryo-EM of mammalian PA28 alpha beta-iCP immunoproteasome reveals a distinct mechanism of proteasome activation by PA28 alpha beta.
Chen, J., Wang, Y., Xu, C., Chen, K., Zhao, Q., Wang, S., Yin, Y., Peng, C., Ding, Z., Cong, Y.(2021) Nat Commun 12: 739-739
- PubMed: 33531497
- DOI: https://doi.org/10.1038/s41467-021-21028-3
- Primary Citation of Related Structures:
7DR6, 7DR7, 7DRW - PubMed Abstract:
The proteasome activator PA28αβ affects MHC class I antigen presentation by associating with immunoproteasome core particles (iCPs). However, due to the lack of a mammalian PA28αβ-iCP structure, how PA28αβ regulates proteasome remains elusive. Here we present the complete architectures of the mammalian PA28αβ-iCP immunoproteasome and free iCP at near atomic-resolution by cryo-EM, and determine the spatial arrangement between PA28αβ and iCP through XL-MS. Our structures reveal a slight leaning of PA28αβ towards the α3-α4 side of iCP, disturbing the allosteric network of the gatekeeper α2/3/4 subunits, resulting in a partial open iCP gate. We find that the binding and activation mechanism of iCP by PA28αβ is distinct from those of constitutive CP by the homoheptameric TbPA26 or PfPA28. Our study sheds lights on the mechanism of enzymatic activity stimulation of immunoproteasome and suggests that PA28αβ-iCP has experienced profound remodeling during evolution to achieve its current level of function in immune response.
Organizational Affiliation:
State Key Laboratory of Molecular Biology, National Center for Protein Science Shanghai, Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai, 200031, China.