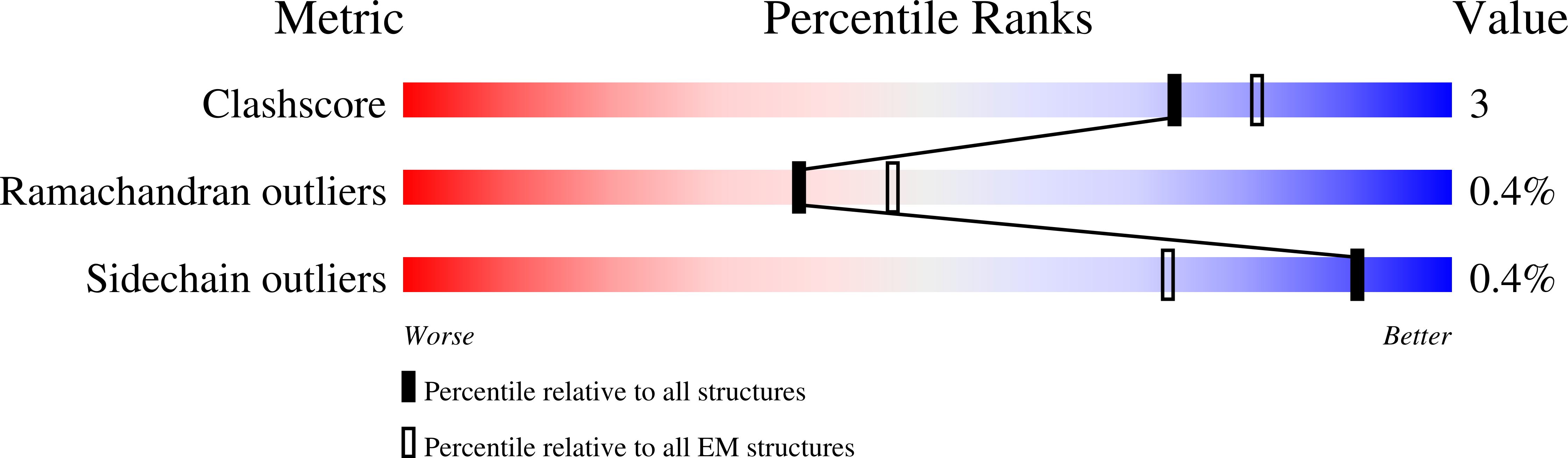A hybrid approach reveals the allosteric regulation of GTP cyclohydrolase I.
Ebenhoch, R., Prinz, S., Kaltwasser, S., Mills, D.J., Meinecke, R., Rubbelke, M., Reinert, D., Bauer, M., Weixler, L., Zeeb, M., Vonck, J., Nar, H.(2020) Proc Natl Acad Sci U S A 117: 31838-31849
- PubMed: 33229582
- DOI: https://doi.org/10.1073/pnas.2013473117
- Primary Citation of Related Structures:
6Z80, 6Z85, 6Z86, 6Z87, 6Z88, 6Z89, 7ACC, 7AL9, 7ALA, 7ALB, 7ALC - PubMed Abstract:
Guanosine triphosphate (GTP) cyclohydrolase I (GCH1) catalyzes the conversion of GTP to dihydroneopterin triphosphate (H2NTP), the initiating step in the biosynthesis of tetrahydrobiopterin (BH4). Besides other roles, BH4 functions as cofactor in neurotransmitter biosynthesis. The BH4 biosynthetic pathway and GCH1 have been identified as promising targets to treat pain disorders in patients. The function of mammalian GCH1s is regulated by a metabolic sensing mechanism involving a regulator protein, GCH1 feedback regulatory protein (GFRP). GFRP binds to GCH1 to form inhibited or activated complexes dependent on availability of cofactor ligands, BH4 and phenylalanine, respectively. We determined high-resolution structures of human GCH1-GFRP complexes by cryoelectron microscopy (cryo-EM). Cryo-EM revealed structural flexibility of specific and relevant surface lining loops, which previously was not detected by X-ray crystallography due to crystal packing effects. Further, we studied allosteric regulation of isolated GCH1 by X-ray crystallography. Using the combined structural information, we are able to obtain a comprehensive picture of the mechanism of allosteric regulation. Local rearrangements in the allosteric pocket upon BH4 binding result in drastic changes in the quaternary structure of the enzyme, leading to a more compact, tense form of the inhibited protein, and translocate to the active site, leading to an open, more flexible structure of its surroundings. Inhibition of the enzymatic activity is not a result of hindrance of substrate binding, but rather a consequence of accelerated substrate binding kinetics as shown by saturation transfer difference NMR (STD-NMR) and site-directed mutagenesis. We propose a dissociation rate controlled mechanism of allosteric, noncompetitive inhibition.
Organizational Affiliation:
Medicinal Chemistry, Boehringer Ingelheim Pharma GmbH & Co. KG, 88397 Biberach an der Riss, Germany.