Optimization of pyrazolo[1,5-a]pyrimidines lead to the identification of a highly selective casein kinase 2 inhibitor.
Kramer, A., Kurz, C.G., Berger, B.T., Celik, I.E., Tjaden, A., Greco, F.A., Knapp, S., Hanke, T.(2020) Eur J Med Chem 208: 112770-112770
- PubMed: 32883634
- DOI: https://doi.org/10.1016/j.ejmech.2020.112770
- Primary Citation of Related Structures:
6YUL, 6YUM - PubMed Abstract:
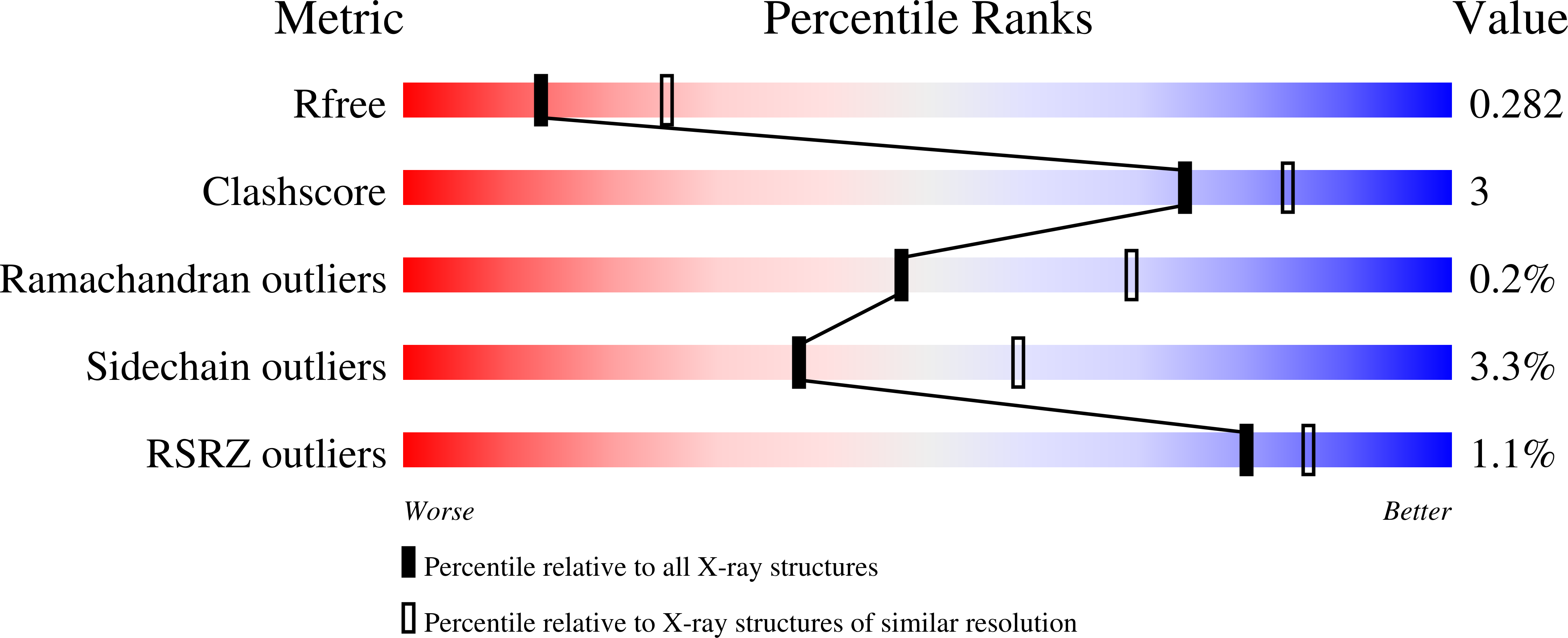
Casein kinase 2 (CK2) is a constitutively expressed serine/threonine kinase that has a large diversity of cellular substrates. Thus, CK2 has been associated with a plethora of regulatory functions and dysregulation of CK2 has been linked to disease development in particular to cancer. The broad implications in disease pathology makes CK2 an attractive target. To date, the most advanced CK2 inhibitor is silmitasertib, which has been investigated in clinical trials for treatment of various cancers, albeit several off-targets for silmitasertib have been described. To ascertain the role of CK2 inhibition in cancer, other disease and normal physiology the development of a selective CK2 inhibitor would be highly desirable. In this study we explored the pyrazolo [1,5-a]pyrimidine hinge-binding moiety for the development of selective CK2 inhibitors. Optimization of this scaffold, which included macrocyclization, led to IC20 (31) a compound that displayed high in vitro potency for CK2 (K D = 12 nM) and exclusive selectivity for CK2. X-ray analysis revealed a canonical type-I binding mode for IC20 (31). However, the polar carboxylic acid moiety that is shared by many CK2 inhibitors including silmitasertib was required for potency but limits the cellular activity of IC20 (31) and the cellular IC 50 dropped to the low micromolar range. In summary, IC20 (31) represents a highly selective and potent inhibitor of CK2, which can be used as a tool compound to study CK2 biology and potential new applications for the treatment of diseases.
Organizational Affiliation:
Institute of Pharmaceutical Chemistry, Max-von-Laue-Straße 9, Goethe University Frankfurt, 60438, Frankfurt, Germany; Structural Genomics Consortium, Buchmann Institute for Molecular Life Sciences (BMLS), Max-von-Laue-Straße 15, 60438, Frankfurt, Germany; Frankfurt Cancer Institute (FCI), Paul-Ehrlich-Straße 42-44, 60596, Frankfurt Am Main, Germany.