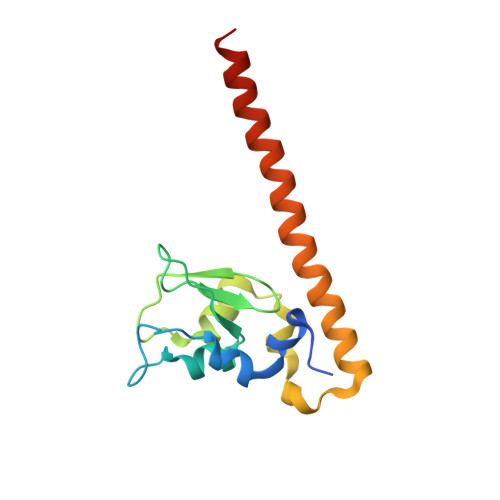
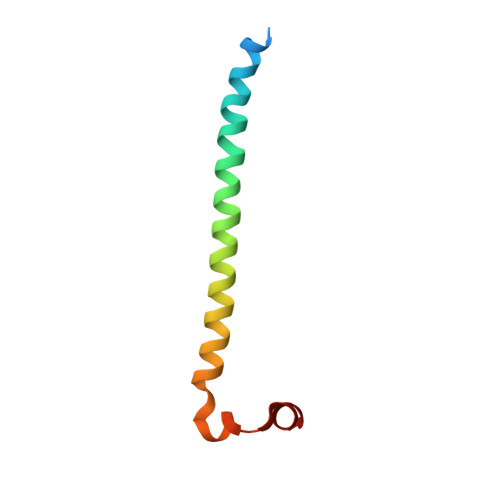
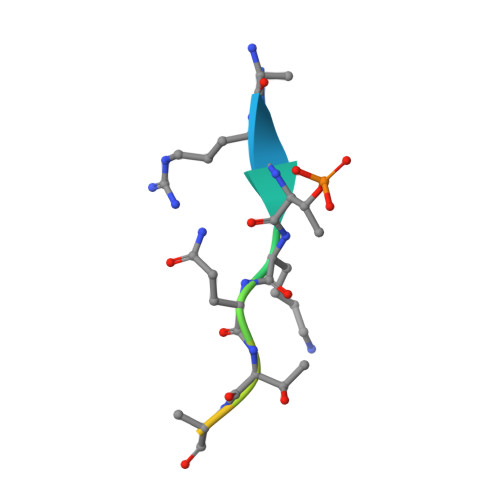
Molecular basis of MKLP2-dependent Aurora B transport from chromatin to the anaphase central spindle.
Serena, M., Bastos, R.N., Elliott, P.R., Barr, F.A.(2020) J Cell Biol 219
- PubMed: 32356865
- DOI: https://doi.org/10.1083/jcb.201910059
- Primary Citation of Related Structures:
6YIE, 6YIF, 6YIH, 6YIP - PubMed Abstract:
The Aurora B chromosomal passenger complex (CPC) is a conserved regulator of mitosis. Its functions require localization first to the chromosome arms and then centromeres in mitosis and subsequently the central spindle in anaphase. Here, we analyze the requirements for core CPC subunits, survivin and INCENP, and the mitotic kinesin-like protein 2 (MKLP2) in targeting to these distinct localizations. Centromere recruitment of the CPC requires interaction of survivin with histone H3 phosphorylated at threonine 3, and we provide a complete structure of this assembly. Furthermore, we show that the INCENP RRKKRR-motif is required for both centromeric localization of the CPC in metaphase and MKLP2-dependent transport in anaphase. MKLP2 and DNA bind competitively to this motif, and INCENP T59 phosphorylation acts as a switch preventing MKLP2 binding in metaphase. In anaphase, CPC binding promotes the microtubule-dependent ATPase activity of MKLP2. These results explain how centromere targeting of the CPC in mitosis is coupled to its movement to the central spindle in anaphase.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, Oxford, UK.