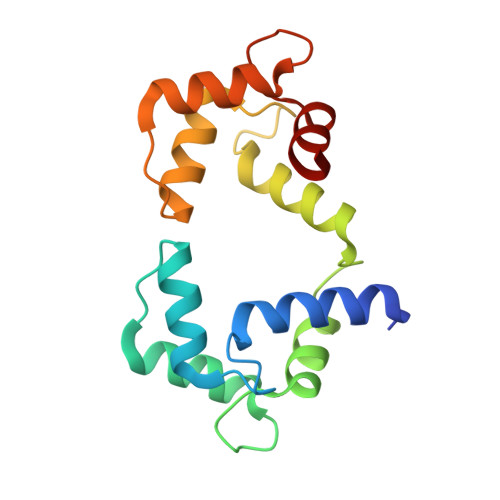
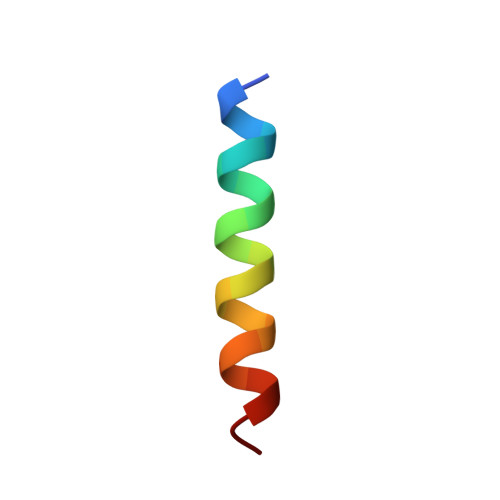
CPVT-associated calmodulin variants N53I and A102V dysregulate Ca2+ signalling via different mechanisms.
Prakash, O., Held, M., McCormick, L.F., Gupta, N., Lian, L.Y., Antonyuk, S., Haynes, L.P., Thomas, N.L., Helassa, N.(2022) J Cell Sci 135
- PubMed: 34888671
- DOI: https://doi.org/10.1242/jcs.258796
- Primary Citation of Related Structures:
6XXF, 6XXX, 6XY3 - PubMed Abstract:
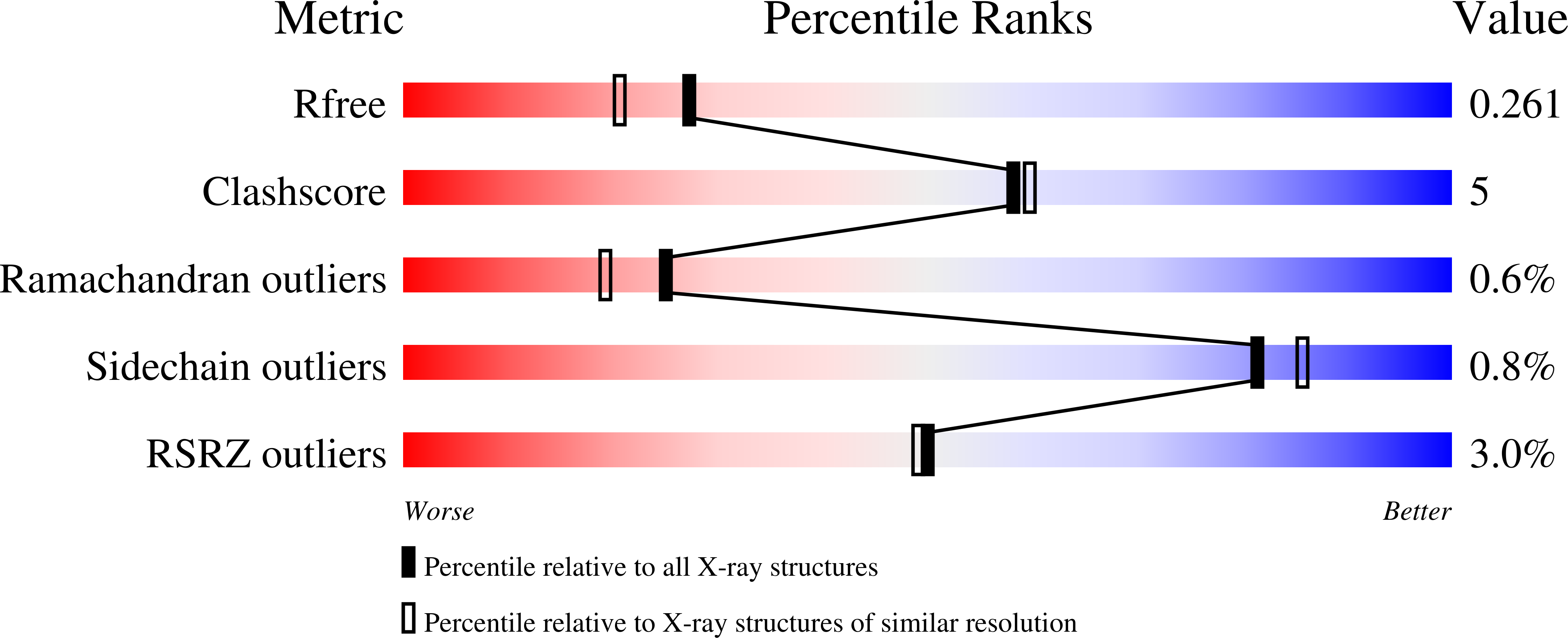
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited condition that can cause fatal cardiac arrhythmia. Human mutations in the Ca2+ sensor calmodulin (CaM) have been associated with CPVT susceptibility, suggesting that CaM dysfunction is a key driver of the disease. However, the detailed molecular mechanism remains unclear. Focusing on the interaction with the cardiac ryanodine receptor (RyR2), we determined the effect of CPVT-associated variants N53I and A102V on the structural characteristics of CaM and on Ca2+ fluxes in live cells. We provide novel data showing that interaction of both Ca2+/CaM-N53I and Ca2+/CaM-A102V with the RyR2 binding domain is decreased. Ca2+/CaM-RyR23583-3603 high-resolution crystal structures highlight subtle conformational changes for the N53I variant, with A102V being similar to wild type (WT). We show that co-expression of CaM-N53I or CaM-A102V with RyR2 in HEK293 cells significantly increased the duration of Ca2+ events; CaM-A102V exhibited a lower frequency of Ca2+ oscillations. In addition, we show that CaMKIIδ (also known as CAMK2D) phosphorylation activity is increased for A102V, compared to CaM-WT. This paper provides novel insight into the molecular mechanisms of CPVT-associated CaM variants and will facilitate the development of strategies for future therapies.
Organizational Affiliation:
Liverpool Centre for Cardiovascular Science, Department of Cardiovascular Science and Metabolic Medicine, Institute of Life Course and Medical Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool L69 3BX, UK.