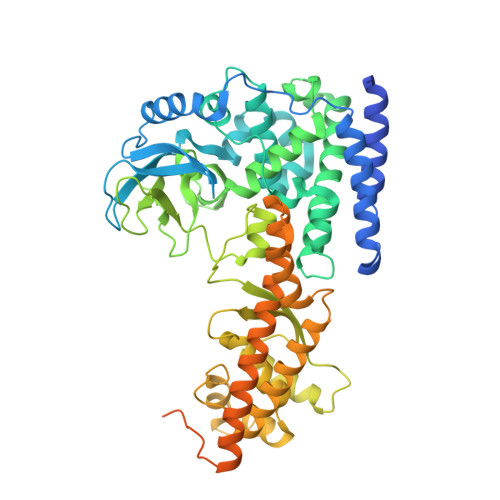
An engineered variant of SETD3 methyltransferase alters target specificity from histidine to lysine methylation.
Dai, S., Horton, J.R., Wilkinson, A.W., Gozani, O., Zhang, X., Cheng, X.(2020) J Biol Chem 295: 2582-2589
- PubMed: 31911441
- DOI: https://doi.org/10.1074/jbc.RA119.012319
- Primary Citation of Related Structures:
6V62, 6V63 - PubMed Abstract:
Most characterized SET domain (SETD) proteins are protein lysine methyltransferases, but SETD3 was recently demonstrated to be a protein ( i.e. actin) histidine-N 3 methyltransferase. Human SETD3 shares a high structural homology with two known protein lysine methyltransferases-human SETD6 and the plant LSMT-but differs in the residues constituting the active site. In the SETD3 active site, Asn 255 engages in a unique hydrogen-bonding interaction with the target histidine of actin that likely contributes to its >1300-fold greater catalytic efficiency ( k cat / K m ) on histidine than on lysine. Here, we engineered active-site variants to switch the SETD3 target specificity from histidine to lysine. Substitution of Asn 255 with phenylalanine (N255F), together with substitution of Trp 273 with alanine (W273A), generated an active site mimicking that of known lysine methyltransferases. The doubly substituted SETD3 variant exhibited a 13-fold preference for lysine over histidine. We show, by means of X-ray crystallography, that the two target nitrogen atoms-the N 3 atom of histidine and the terminal ϵ-amino nitrogen of lysine-occupy the same position and point toward and are within a short distance of the incoming methyl group of SAM for a direct methyl transfer during catalysis. In contrast, SETD3 and its Asn 255 substituted derivatives did not methylate glutamine (another potentially methylated amino acid). However, the glutamine-containing peptide competed with the substrate peptide, and glutamine bound in the active site, but too far away from SAM to be methylated. Our results provide insight into the structural parameters defining the target amino acid specificity of SET enzymes.
Organizational Affiliation:
Department of Epigenetics and Molecular Carcinogenesis, University of Texas M.D. Anderson Cancer Center, Houston, Texas 77030.