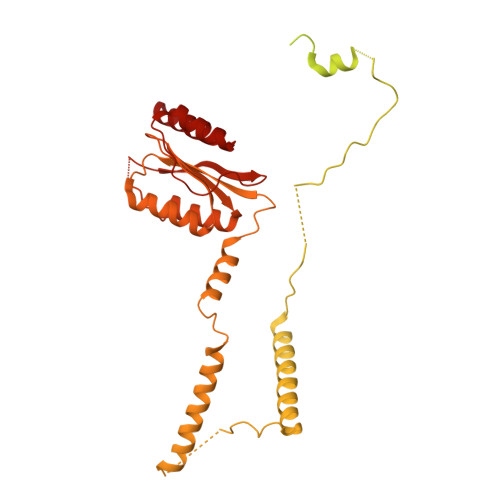
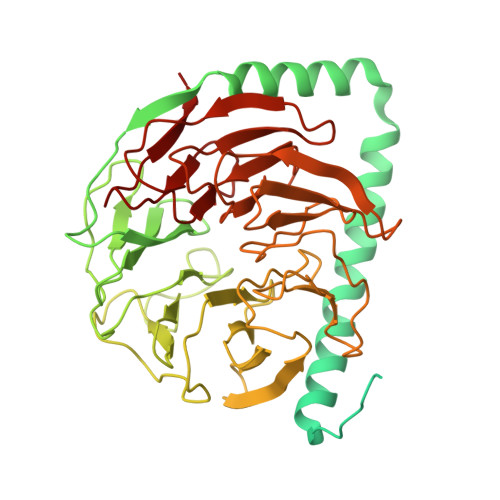
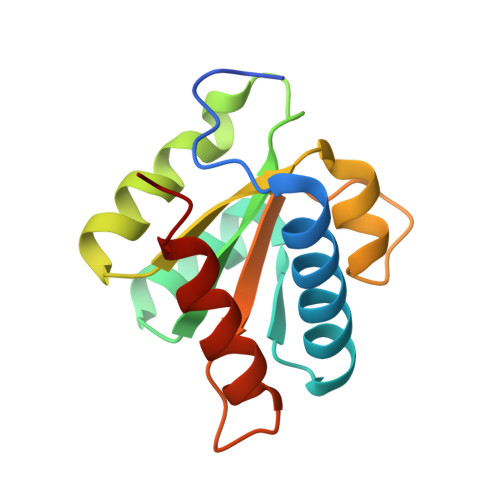
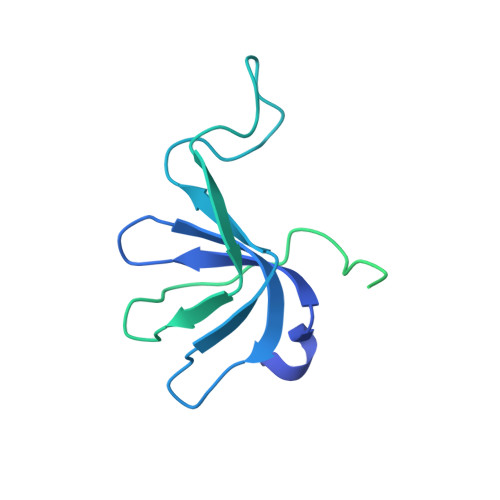
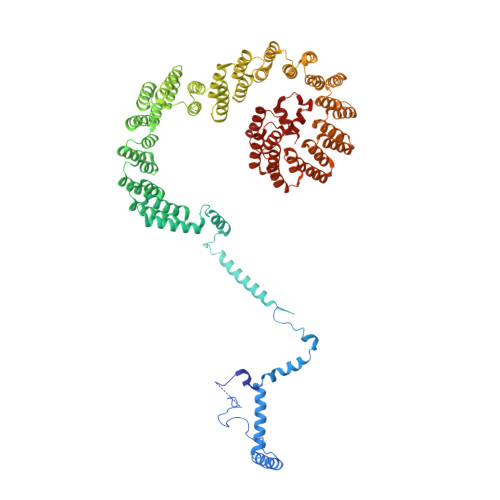
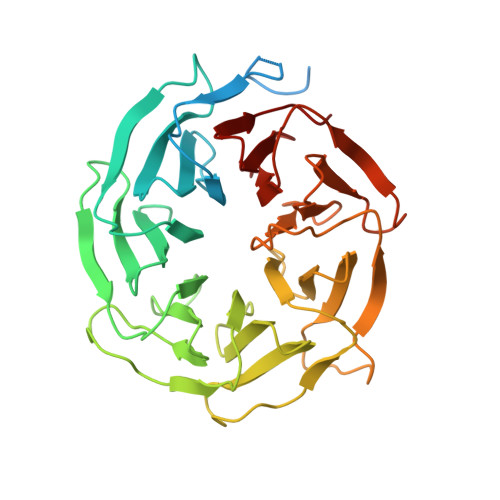
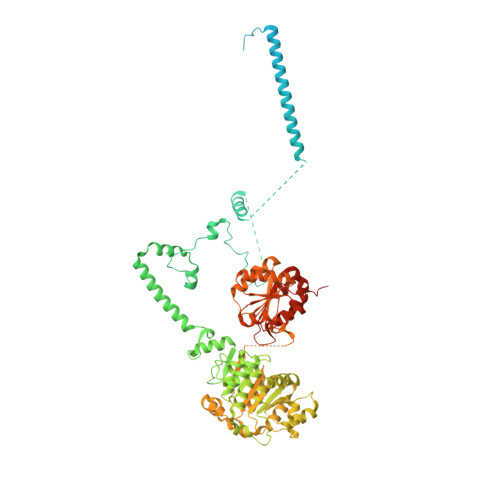
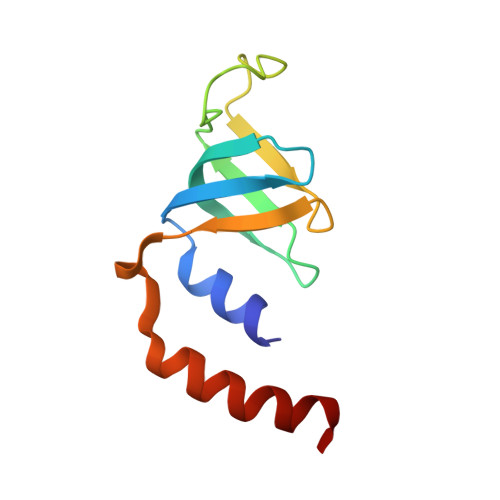
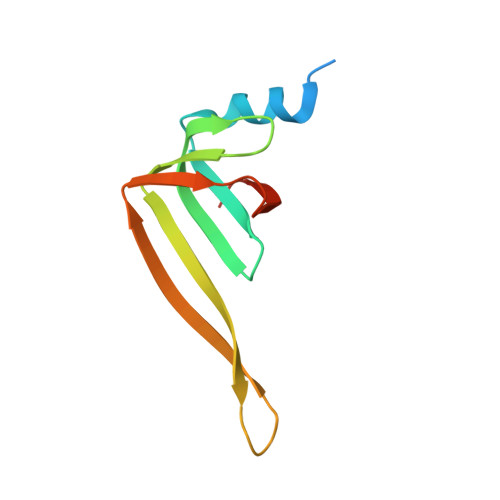
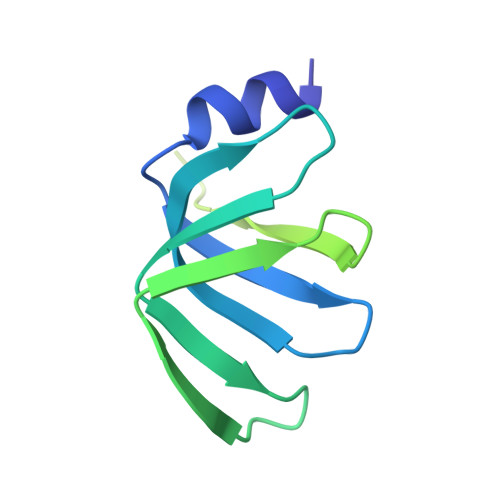
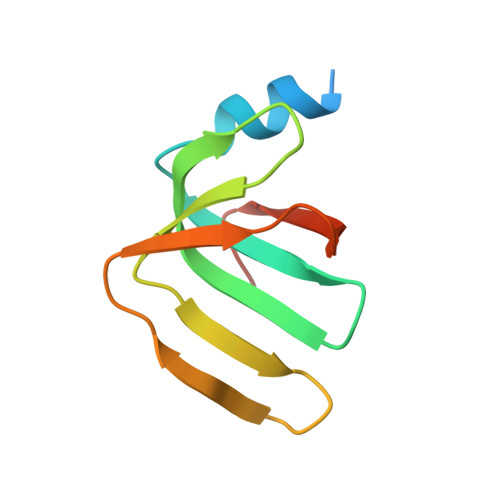
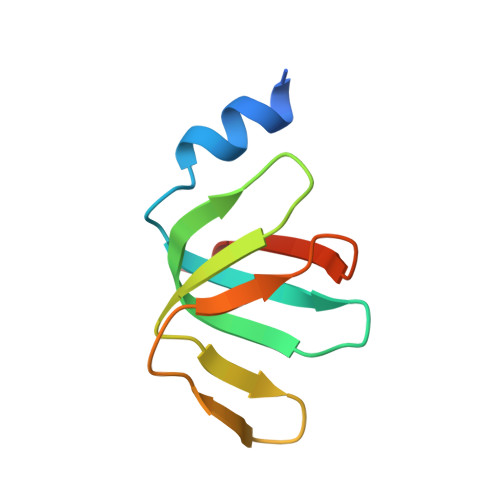
Mechanism of 5' splice site transfer for human spliceosome activation.
Charenton, C., Wilkinson, M.E., Nagai, K.(2019) Science 364: 362-367
- PubMed: 30975767
- DOI: https://doi.org/10.1126/science.aax3289
- Primary Citation of Related Structures:
6QW6, 6QX9 - PubMed Abstract:
The prespliceosome, comprising U1 and U2 small nuclear ribonucleoproteins (snRNPs) bound to the precursor messenger RNA 5' splice site (5'SS) and branch point sequence, associates with the U4/U6.U5 tri-snRNP to form the fully assembled precatalytic pre-B spliceosome. Here, we report cryo-electron microscopy structures of the human pre-B complex captured before U1 snRNP dissociation at 3.3-angstrom core resolution and the human tri-snRNP at 2.9-angstrom resolution. U1 snRNP inserts the 5'SS-U1 snRNA helix between the two RecA domains of the Prp28 DEAD-box helicase. Adenosine 5'-triphosphate-dependent closure of the Prp28 RecA domains releases the 5'SS to pair with the nearby U6 ACAGAGA-box sequence presented as a mobile loop. The structures suggest that formation of the 5'SS-ACAGAGA helix triggers remodeling of an intricate protein-RNA network to induce Brr2 helicase relocation to its loading sequence in U4 snRNA, enabling Brr2 to unwind the U4/U6 snRNA duplex to allow U6 snRNA to form the catalytic center of the spliceosome.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge CB2 0QH, UK. ccharent@mrc-lmb.cam.ac.uk mwilkin@mrc-lmb.cam.ac.uk kn@mrc-lmb.cam.ac.uk.