A tunable LIC1-adaptor interaction modulates dynein activity in a cargo-specific manner.
Lee, I.G., Cason, S.E., Alqassim, S.S., Holzbaur, E.L.F., Dominguez, R.(2020) Nat Commun 11: 5695-5695
- PubMed: 33173051
- DOI: https://doi.org/10.1038/s41467-020-19538-7
- Primary Citation of Related Structures:
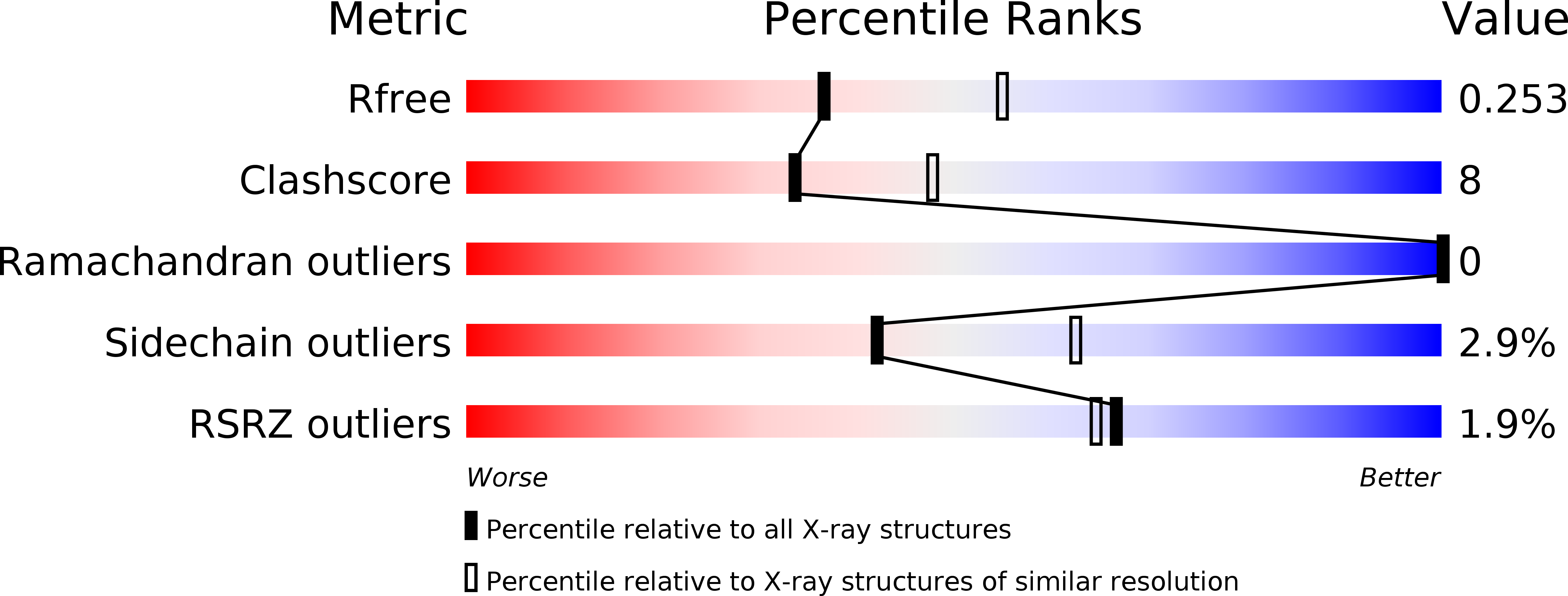
6PSD, 6PSE - PubMed Abstract:
Cytoplasmic dynein-1 (dynein) is the motor responsible for most retrograde transport of cargoes along microtubules in eukaryotic cells, including organelles, mRNA and viruses. Cargo selectivity and activation of processive motility depend on a group of so-called "activating adaptors" that link dynein to its general cofactor, dynactin, and cargoes. The mechanism by which these adaptors regulate dynein transport is poorly understood. Here, based on crystal structures, quantitative binding studies, and in vitro motility assays, we show that BICD2, CRACR2a, and HOOK3, representing three subfamilies of unrelated adaptors, interact with the same amphipathic helix of the dynein light intermediate chain-1 (LIC1). While the hydrophobic character of the interaction is conserved, the three adaptor subfamilies use different folds (coiled-coil, EF-hand, HOOK domain) and different surface contacts to bind the LIC1 helix with affinities ranging from 1.5 to 15.0 μM. We propose that a tunable LIC1-adaptor interaction modulates dynein's motility in a cargo-specific manner.
Organizational Affiliation:
Department of Physiology and Pennsylvania Muscle Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA.