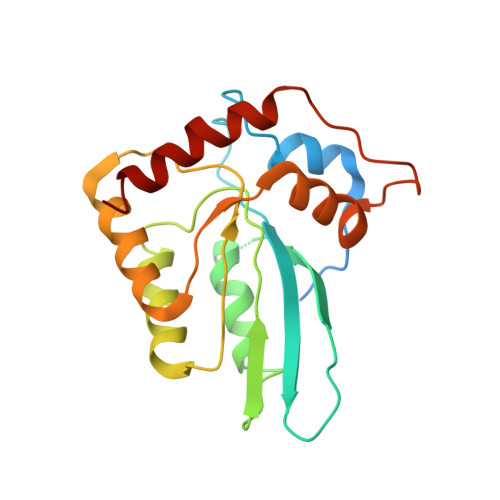
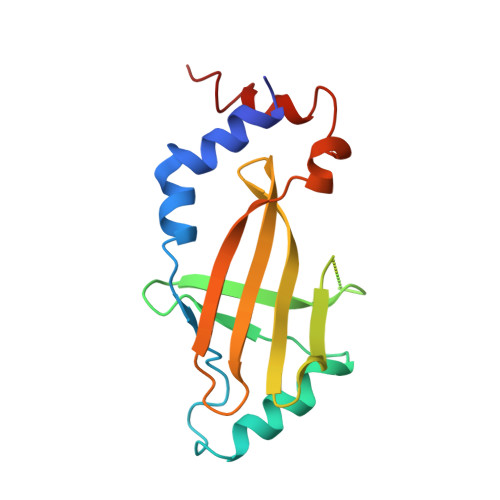
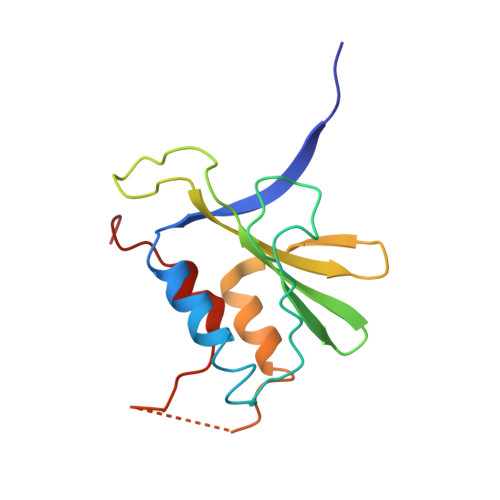
Structural basis of antagonism of human APOBEC3F by HIV-1 Vif.
Hu, Y., Desimmie, B.A., Nguyen, H.C., Ziegler, S.J., Cheng, T.C., Chen, J., Wang, J., Wang, H., Zhang, K., Pathak, V.K., Xiong, Y.(2019) Nat Struct Mol Biol 26: 1176-1183
- PubMed: 31792451
- DOI: https://doi.org/10.1038/s41594-019-0343-6
- Primary Citation of Related Structures:
6NIL - PubMed Abstract:
HIV-1 virion infectivity factor (Vif) promotes degradation of the antiviral APOBEC3 (A3) proteins through the host ubiquitin-proteasome pathway to enable viral immune evasion. Disrupting Vif-A3 interactions to reinstate the A3-catalyzed suppression of human immunodeficiency virus type 1 (HIV-1) replication is a potential approach for antiviral therapeutics. However, the molecular mechanisms by which Vif recognizes A3 proteins remain elusive. Here we report a cryo-EM structure of the Vif-targeted C-terminal domain of human A3F in complex with HIV-1 Vif and the cellular cofactor core-binding factor beta (CBFβ) at 3.9-Å resolution. The structure shows that Vif and CBFβ form a platform to recruit A3F, revealing a direct A3F-recruiting role of CBFβ beyond Vif stabilization, and captures multiple independent A3F-Vif interfaces. Together with our biochemical and cellular studies, our structural findings establish the molecular determinants that are critical for Vif-mediated neutralization of A3F and provide a comprehensive framework of how HIV-1 Vif hijacks the host protein degradation machinery to counteract viral restriction by A3F.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT, USA.