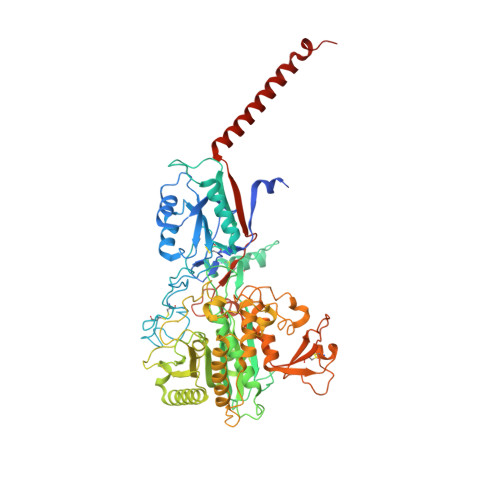
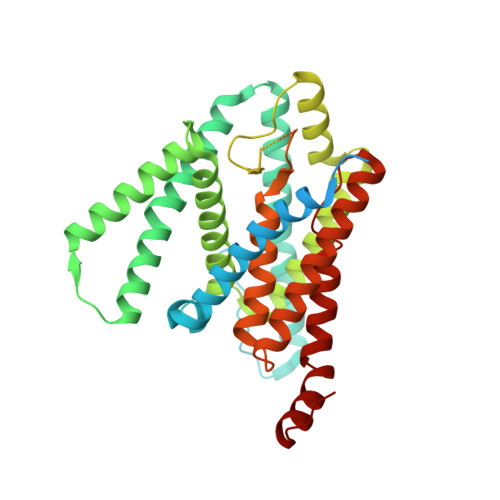
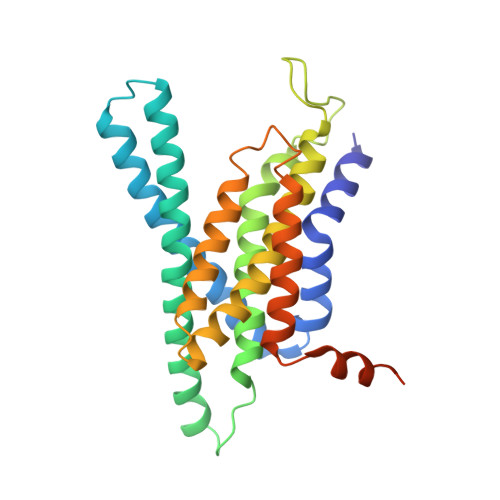
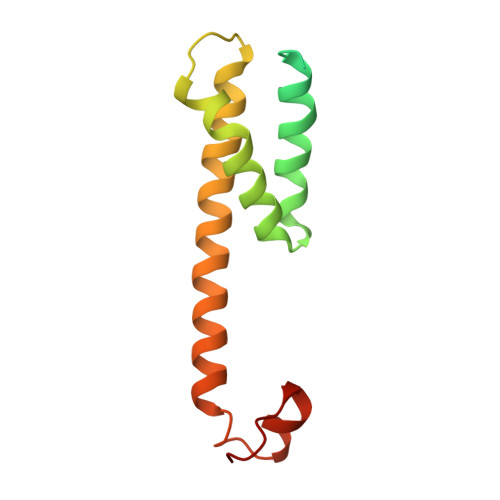
Structural basis of gamma-secretase inhibition and modulation by small molecule drugs.
Yang, G., Zhou, R., Guo, X., Yan, C., Lei, J., Shi, Y.(2021) Cell 184: 521-533.e14
- PubMed: 33373587
- DOI: https://doi.org/10.1016/j.cell.2020.11.049
- Primary Citation of Related Structures:
6LQG, 6LR4, 7C9I, 7D8X - PubMed Abstract:
Development of γ-secretase inhibitors (GSIs) and modulators (GSMs) represents an attractive therapeutic opportunity for Alzheimer's disease (AD) and cancers. However, how these GSIs and GSMs target γ-secretase has remained largely unknown. Here, we report the cryoelectron microscopy (cryo-EM) structures of human γ-secretase bound individually to two GSI clinical candidates, Semagacestat and Avagacestat, a transition state analog GSI L685,458, and a classic GSM E2012, at overall resolutions of 2.6-3.1 Å. Remarkably, each of the GSIs occupies the same general location on presenilin 1 (PS1) that accommodates the β strand from amyloid precursor protein or Notch, interfering with substrate recruitment. L685,458 directly coordinates the two catalytic aspartate residues of PS1. E2012 binds to an allosteric site of γ-secretase on the extracellular side, potentially explaining its modulating activity. Structural analysis reveals a set of shared themes and variations for inhibitor and modulator recognition that will guide development of the next-generation substrate-selective inhibitors.
Organizational Affiliation:
Beijing Advanced Innovation Center for Structural Biology and Research Center for Biological Structure, Tsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing 100084, China; State Key Laboratory for Agrobiotechnology, College of Biological Sciences, China Agricultural University, Beijing 100193, China.