F-actin disassembly factor MICAL1 binding to Myosin Va mediates cargo unloading during cytokinesis.
Niu, F., Sun, K., Wei, W., Yu, C., Wei, Z.(2020) Sci Adv 6
- PubMed: 33158857
- DOI: https://doi.org/10.1126/sciadv.abb1307
- Primary Citation of Related Structures:
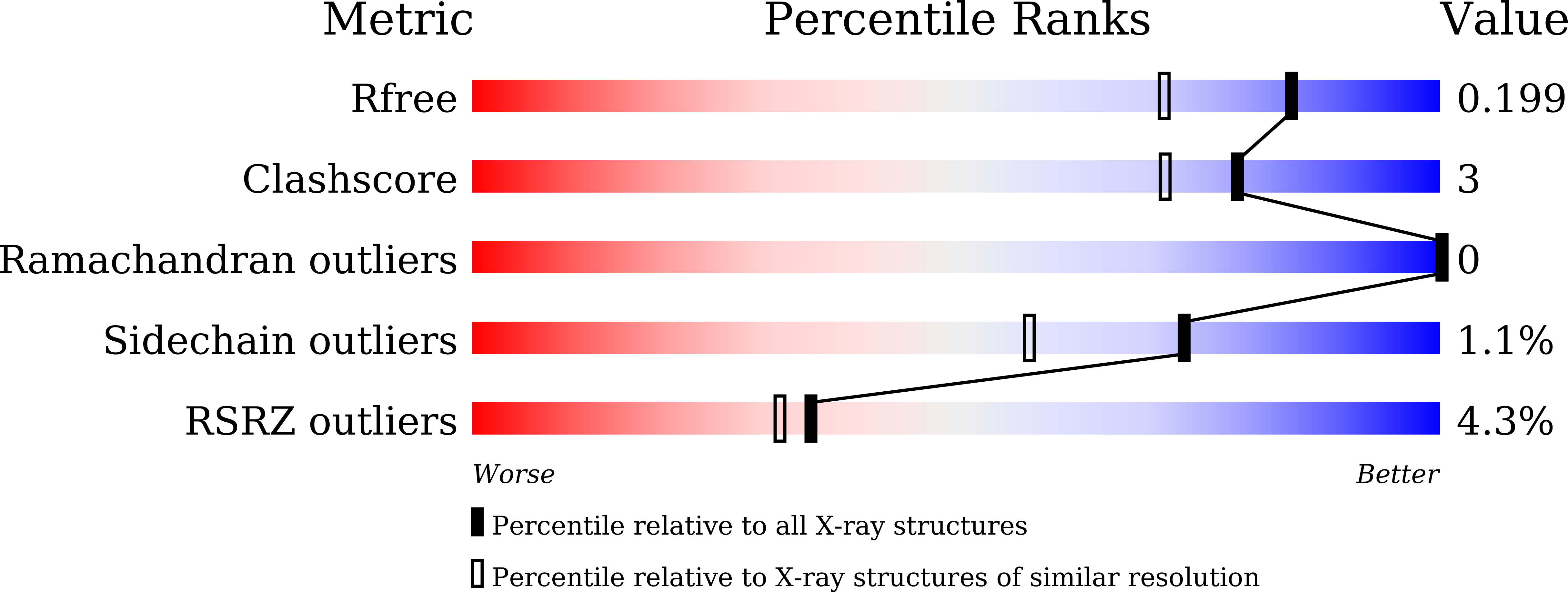
6KU0 - PubMed Abstract:
Motor-mediated intracellular trafficking requires motors to position cargoes at proper locations. Myosin Va (MyoVa), an actin-based motor, is a classic model for studying cargo transport. However, the molecular basis underlying cargo unloading in MyoVa-mediated transport has remained enigmatic. We have identified MICAL1, an F-actin disassembly regulator, as a binding partner of MyoVa and shown that MICAL1-MyoVa interaction is critical for localization of MyoVa at the midbody. By binding to MICAL1, MyoVa-mediated transport is terminated, resulting in vesicle unloading at the midbody for efficient cytokinesis. The MyoVa/MICAL1 complex structure reveals that MICAL1 and F-actin assembly factors, Spires, share an overlapped binding surface on MyoVa, suggesting a regulatory role of F-actin dynamics in cargo unloading. Down-regulating F-actin disassembly by a MICAL1 mutant significantly reduces MyoVa and vesicles accumulating at the midbody. Collectively, our findings demonstrate that MyoVa binds to MICAL1 at the midbody destination and triggers F-actin disassembly to unload the vesicle cargo.
Organizational Affiliation:
Department of Biology, Southern University of Science and Technology, Shenzhen, Guangdong, China.