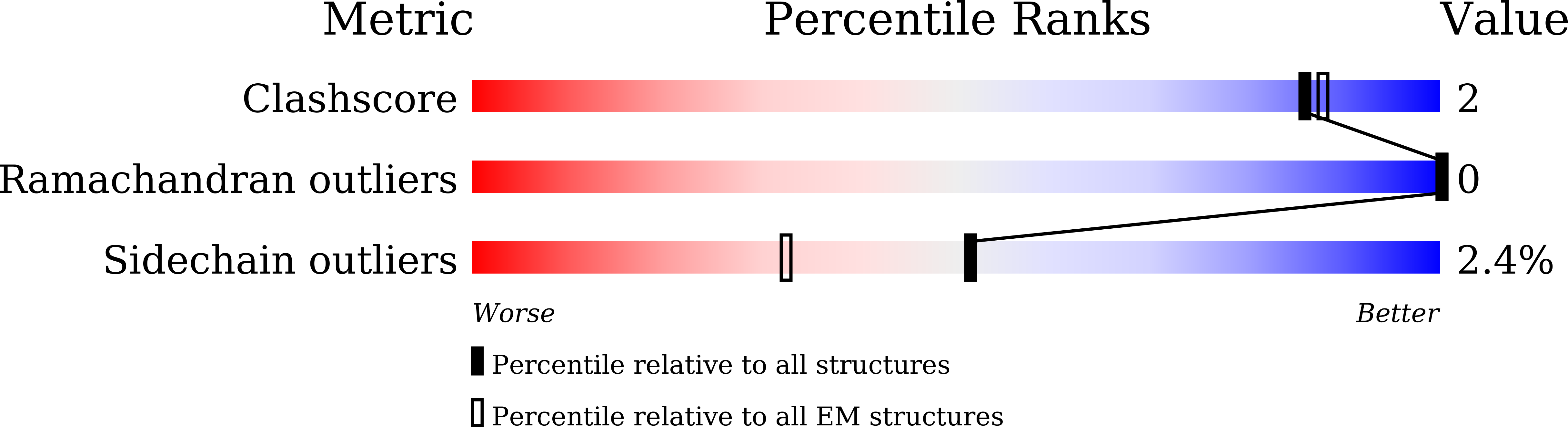Structural basis for distinct inflammasome complex assembly by human NLRP1 and CARD8.
Gong, Q., Robinson, K., Xu, C., Huynh, P.T., Chong, K.H.C., Tan, E.Y.J., Zhang, J., Boo, Z.Z., Teo, D.E.T., Lay, K., Zhang, Y., Lim, J.S.Y., Goh, W.I., Wright, G., Zhong, F.L., Reversade, B., Wu, B.(2021) Nat Commun 12: 188-188
- PubMed: 33420028
- DOI: https://doi.org/10.1038/s41467-020-20319-5
- Primary Citation of Related Structures:
6K7V, 6K8J, 6K99, 6K9F - PubMed Abstract:
Nod-like receptor (NLR) proteins activate pyroptotic cell death and IL-1 driven inflammation by assembling and activating the inflammasome complex. Closely related sensor proteins NLRP1 and CARD8 undergo unique auto-proteolysis-dependent activation and are implicated in auto-inflammatory diseases; however, their mechanisms of activation are not understood. Here we report the structural basis of how the activating domains (FIIND UPA -CARD) of NLRP1 and CARD8 self-oligomerize to assemble distinct inflammasome complexes. Recombinant FIIND UPA -CARD of NLRP1 forms a two-layered filament, with an inner core of oligomerized CARD surrounded by an outer ring of FIIND UPA . Biochemically, self-assembled NLRP1-CARD filaments are sufficient to drive ASC speck formation in cultured human cells-a process that is greatly enhanced by NLRP1-FIIND UPA which forms oligomers in vitro. The cryo-EM structures of NLRP1-CARD and CARD8-CARD filaments, solved here at 3.7 Å, uncover unique structural features that enable NLRP1 and CARD8 to discriminate between ASC and pro-caspase-1. In summary, our findings provide structural insight into the mechanisms of activation for human NLRP1 and CARD8 and reveal how highly specific signaling can be achieved by heterotypic CARD interactions within the inflammasome complexes.
Organizational Affiliation:
School of Biological Sciences, Nanyang Technological University, Singapore, 637551, Singapore.