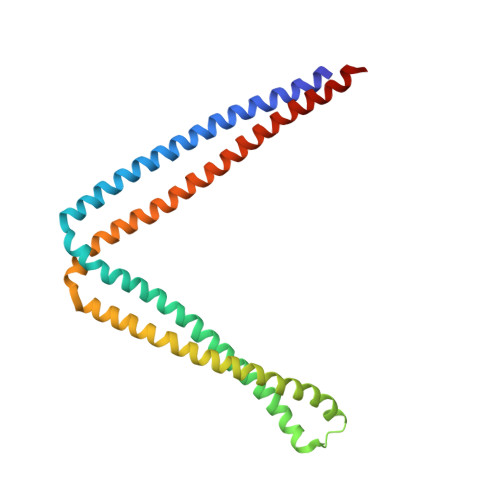Structural basis of guanine nucleotide exchange for Rab11 by SH3BP5.
Goto-Ito, S., Morooka, N., Yamagata, A., Sato, Y., Sato, K., Fukai, S.(2019) Life Sci Alliance 2
- PubMed: 30872413
- DOI: https://doi.org/10.26508/lsa.201900297
- Primary Citation of Related Structures:
6IXE, 6IXF, 6IXG, 6IXV - PubMed Abstract:
The Rab GTPase family is a major regulator of membrane traffic in eukaryotic cells. The Rab11 subfamily plays important roles in specific trafficking events such as exocytosis, endosomal recycling, and cytokinesis. SH3BP5 and SH3BP5-like (SH3BP5L) proteins have recently been found to serve as guanine nucleotide exchange factors (GEF) for Rab11. Here, we report the crystal structures of the SH3BP5 GEF domain alone and its complex with Rab11a. SH3BP5 exhibits a V-shaped structure comprising two coiled coils. The coiled coil composed of α1, and α4 is solely responsible for the Rab11a binding and GEF activity. SH3BP5 pulls out and deforms switch I of Rab11a so as to facilitate the GDP release from Rab11a. SH3BP5 interacts with the N-terminal region, switch I, interswitch, and switch II of Rab11a. SH3BP5 and SH3BP5L localize to Rab11-positive recycling endosomes and show GEF activity for all of the Rab11 family but not for Rab14. Fluorescence-based GEF assays combined with site-directed mutagenesis reveal the essential interactions between SH3BP5 and Rab11 family proteins for the GEF reaction on recycling endosomes.
Organizational Affiliation:
Institute for Quantitative Biosciences, The University of Tokyo, Tokyo, Japan.