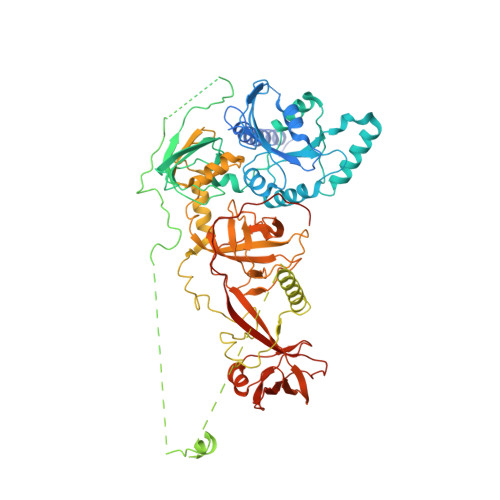
Visualizing late states of human 40S ribosomal subunit maturation.
Ameismeier, M., Cheng, J., Berninghausen, O., Beckmann, R.(2018) Nature 558: 249-253
- PubMed: 29875412
- DOI: https://doi.org/10.1038/s41586-018-0193-0
- Primary Citation of Related Structures:
6G18, 6G4S, 6G4W, 6G51, 6G53, 6G5H, 6G5I - PubMed Abstract:
The formation of eukaryotic ribosomal subunits extends from the nucleolus to the cytoplasm and entails hundreds of assembly factors. Despite differences in the pathways of ribosome formation, high-resolution structural information has been available only from fungi. Here we present cryo-electron microscopy structures of late-stage human 40S assembly intermediates, representing one state reconstituted in vitro and five native states that range from nuclear to late cytoplasmic. The earliest particles reveal the position of the biogenesis factor RRP12 and distinct immature rRNA conformations that accompany the formation of the 40S subunit head. Molecular models of the late-acting assembly factors TSR1, RIOK1, RIOK2, ENP1, LTV1, PNO1 and NOB1 provide mechanistic details that underlie their contribution to a sequential 40S subunit assembly. The NOB1 architecture displays an inactive nuclease conformation that requires rearrangement of the PNO1-bound 3' rRNA, thereby coordinating the final rRNA folding steps with site 3 cleavage.
Organizational Affiliation:
Gene Center Munich and Center of Integrated Protein Science-Munich (CiPS-M), Department of Biochemistry, University of Munich, Munich, Germany.