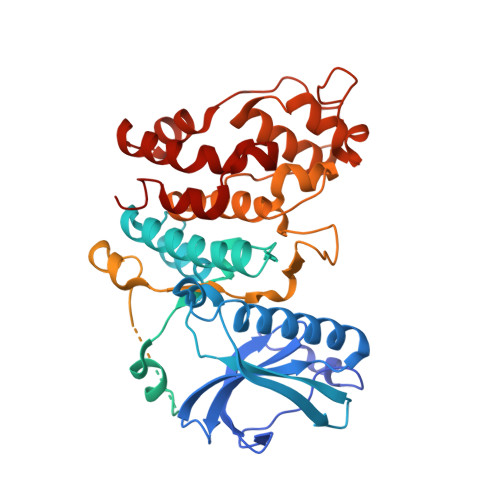
Molecular Mechanism of SR Protein Kinase 1 Inhibition by the Herpes Virus Protein ICP27.
Tunnicliffe, R.B., Hu, W.K., Wu, M.Y., Levy, C., Mould, A.P., McKenzie, E.A., Sandri-Goldin, R.M., Golovanov, A.P.(2019) mBio 10
- PubMed: 31641093
- DOI: https://doi.org/10.1128/mBio.02551-19
- Primary Citation of Related Structures:
6FAD - PubMed Abstract:
Serine-arginine (SR) protein kinase 1 (SRPK1) catalyzes the phosphorylation of SR proteins, which are a conserved family of splicing factors that contain a domain rich in arginine and serine repeats. SR proteins play important roles in constitutive pre-mRNA splicing and are also important regulators of alternative splicing. During herpes simplex virus infection, SRPK1 is inactivated and its cellular distribution is markedly altered by interaction with the viral protein ICP27, resulting in hypophosphorylation of SR proteins. Mutational analysis previously showed that the RGG box motif of ICP27 is required for interaction with SRPK1; however, the mechanism for the inhibition and the exact role of the RGG box was unknown. Here, we used solution nuclear magnetic resonance (NMR) spectroscopy and isothermal titration calorimetry (ITC) to demonstrate that the isolated peptide comprising the RGG box of ICP27 binds to SRPK1 with high affinity, competing with a native substrate, the SR repeat region of SR protein SRSF1. We determined the crystal structure of the complex between SRPK1 and an RGG box peptide, which revealed that the viral peptide binds to the substrate docking groove, mimicking the interactions of SR repeats. Site-directed mutagenesis within the RGG box further confirmed the importance of selected arginine residues for interaction, relocalization, and inhibition of SRPK1 in vivo Together these data reveal the molecular mechanism of the competitive inhibition of cellular SRPK1 by viral ICP27, which modulates SRPK1 activity. IMPORTANCE Serine arginine (SR) proteins are a family of mRNA regulatory proteins that can modulate spliceosome association with different splice sites and therefore regulate alternative splicing. Phosphorylation within SR proteins is necessary for splice-site recognition, and this is catalyzed by SR protein kinase 1 (SRPK1). The herpes simplex virus (HSV-1) protein ICP27 has been shown previously to interact with and downregulate SRPK1 activity in vivo ; however, the molecular mechanism for this interaction and inhibition was unknown. Here, we demonstrate that the isolated peptide fragment of ICP27 containing RGG box binds to SRPK1 with high affinity, and competes with a native cellular substrate. Elucidation of the SRPK1-RGG box crystal structure further showed that a short palindromic RGRRRGR sequence binds in the substrate docking groove of SRPK1, mimicking the binding of SR repeats of substrates. These data reveal how the viral protein ICP27 inactivates SRPK1, promoting hypophosphorylation of proteins regulating splicing.
Organizational Affiliation:
Manchester Institute of Biotechnology and Department of Chemistry, Faculty of Science and Engineering, The University of Manchester, Manchester, United Kingdom.